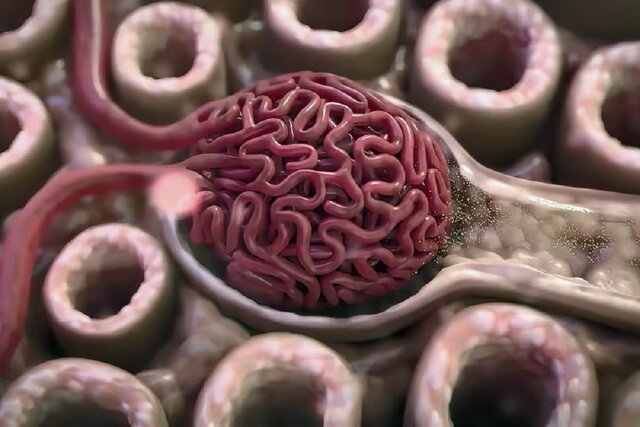
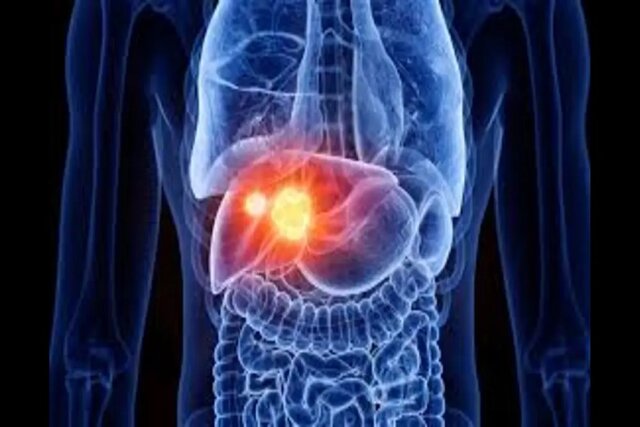
Low-cost cancer treatment with a device the size of a microwave. A Belgian biotech company is testing a device that produces cancer drugs in hospitals, reducing waiting times and the cost of treatment.
Low-cost cancer treatment with a device the size of a microwave
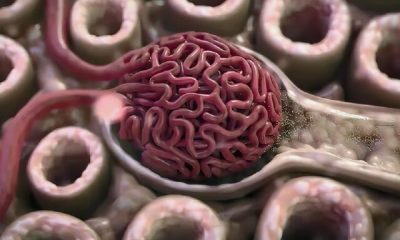
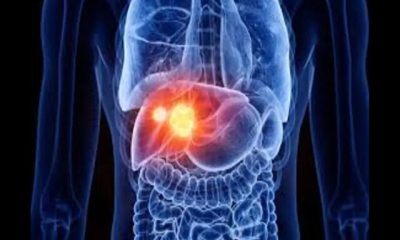
In this article we’re going to read about Low-cost cancer treatment with a device the size of a microwave. When cancer treatment with a chimeric antigen receptor T cell, or CAR-T, works, it can seem miraculous. About half of leukemia and lymphoma patients, and about a third of myeloma patients, get a complete cure with a single injection of immune T cells that have been genetically modified to find and kill cancer in the blood. CAR-T treatment for acute lymphoblastic leukemia, which is the most common type of childhood cancer, has shown a cure rate of up to 90%. The first two patients treated with CAR-T in 2010 were adult men suffering from end-stage acute lymphoblastic leukemia. They were still in remission a decade after treatment.
Since 2017, the US Food and Drug Administration (FDA) has approved a total of six CAR-T therapies, all for blood cancers. Many studies have been conducted with the aim of using CAR-T therapy on solid tumors, but none are yet at the clinical trial stage. Two of these treatments, known as “Yescarta” and “Tecartus”, have earned 1.5 billion dollars for Kite Pharma in 2022 alone. Until recently, CAR-T therapies were mainly considered a last resort for patients who have tried other drugs, but CAR-T therapy can be used earlier in the treatment process and is likely to have a big impact. Last year, Yescarta was approved as a second-line treatment for large B-cell lymphoma. Despite this, drug makers are currently facing a problem.
A survey conducted in 2022 by Mayo Clinic researchers found that the average time on the waiting list for CAR-T treatment was six months, and only a quarter of patients eventually received it. Another quarter was able to enter a clinical trial for treatments that have yet to be approved. In the past few years, Bristol Myers Squibb, Kite Pharma, and Novartis have all experienced manufacturing problems with their CAR-T therapies. Johnson & Johnson (J&J) and Legend Biotech (Legend Biotech) decided in March to stop launching their CAR-T therapy, Carvycti, in the UK due to production constraints.
Unlike conventional drugs, autologous CAR-T injections are living drugs that are customized for each patient. Blood sampling of patients is usually done in a hospital or special center. Once isolated, the T cells are shipped frozen to a biomanufacturing facility where they are genetically reprogrammed to express a tumor-seeking molecule called a chimeric antigen receptor (CAR) on their surface. The modified cells are placed in an incubator for days or weeks until their numbers increase enough to create a therapeutic dose. After several stages of quality testing, the modified CAR-T cells are frozen and returned to the hospital to be injected into the patient. This process usually takes a minimum of two weeks and a maximum of eight weeks.
Current CAR-T treatments cost between $300,000 and $400,000. Travis Young, vice president of the biology department at the non-profit California Biomedical Research Institute (Calibr), said: “The reason for the high cost of treatment is that the production process must be highly controlled at every point of it.” This requires a trained technician, clean rooms, and infrastructure for transportation and freezing. The most important time is pre-release testing to ensure product sterility and potency. There are many possibilities for problems. The supply chain is still in its infancy, and it’s not just about the infrastructure, it’s about the number of people who need to be trained to do the job.
Companies are tackling these challenges in a variety of ways, aiming to reduce the complexity, time, and cost of delivering CAR-T therapies to more patients. One of the more unlikely competitors is Belgium-based Galapagos NV, which last June announced a bold plan to produce these expensive treatments faster and more cost-effectively. The program proposes developing treatments not in a centralized location, but at the point of care, using a small automated device the size of a home microwave.
Galapagos had no prior experience with CAR-T therapy and had only marketed one product in Europe, the UK, and Japan since its inception in 1999. This product was the drug “Jyseleca” for the treatment of ulcerative colitis and rheumatoid arthritis, whose sales in 2022 were reported to be equal to 95 million dollars. The drug has recently undergone a series of clinical trials, but it has one special advantage: Paul Stoffels, the new CEO of Galapagos and former chief scientific officer of Johnson & Johnson.
When Stoffels left J&J at the end of 2021, he had one of the most enviable track records in the pharmaceutical industry. This Belgian-born doctor and specialist in infectious diseases during his training was in charge of the groups that produced 25 new drugs; including two successful cancer drugs, breakthrough treatments for HIV and tuberculosis, and vaccines for Ebola and Covid-19. Although Carvycti was approved a few months after Stoffels left, it was developed under his watch. During Stoffels’ tenure, J&J’s pharmaceutical sales more than doubled from $22.5 billion in 2009 to $45.6 billion in 2020. Seven of the drugs developed under Stoffels’ supervision have been added to the “World Health Organization’s” (WHO) list of essential drugs, which means that they are considered necessary to maintain health.
Stoffels’ stint in the Galapagos gives him the opportunity to demonstrate his ability in a larger company. Immediately after taking over as CEO last April, Stoffels orchestrated a major pivot, buying two startups working on different aspects of CAR-T therapies and manufacturing them, and four months later hired 200 people working on drug programs. They were working older, fired.
Galapagos sets up manufacturing units at each of its partner hospitals, which includes training people, installing equipment, and validating the manufacturing process, Stoffels explained about the process. This is a new approach but much simpler than centralized manufacturing. In centralized manufacturing, you have to invest several hundred million dollars in a building, hire between 500 and 1,000 people, train them, and produce the drug there, but for us, the heavy lifting of this technology has already been done.
From a biological perspective, using diseased cells that have never been frozen has advantages that affect cell health. Newly generated CAR-T cells, after re-injection into the patient, show robust and consistent growth, which helps minimize a common side effect of CAR-T therapy called cytokine release syndrome, Stoffels continued. This syndrome is an aggressive reaction to immunotherapy that causes fever, nausea, and fatigue. The fact that the treatment can be done in seven days allows people with a very short life expectancy to receive this type of treatment. The first patient treated with CAR-T, who came to the hospital with acute respiratory distress syndrome and severe tumor recurrence, is still in excellent condition. This could never have been done with CAR-T the old way and in one centralized location.
Read More: Scientists discovered the secret of DNA’s X shape
Decentralization, simplification, and automation of the entire process will significantly reduce CAR-T costs, Stoffels added. The time required and the amount of work that needs to be done make CAR-T treatments expensive. If you put four or five systems in a hospital room, you can treat 200 patients a year using only a small staff.
Galapagos will not be immune to shortages of chemical reagents and other raw materials that affect other companies. “All the challenges are compounded by not having trained technicians to do the work,” Travis Young said. Technicians don’t need a lot of training because the systems require a lot less manipulation, but whenever you distribute these systems across hospital centers, you lose some control over them all.
After all, Stoffels has made the impossible possible before, and he’s done it many times, and he doesn’t seem to be giving up. He added: “I have worked all my life trying to get access to medicines.” This work is also such a mission. New science allows us to do new, difficult, and different things, and if you don’t start, you will never reach the goal.



 Technology9 months ago
Technology9 months ago


 Technology10 months ago
Technology10 months ago


 Technology9 months ago
Technology9 months ago


 Technology10 months ago
Technology10 months ago

 Humans1 year ago
Humans1 year ago


 AI1 year ago
AI1 year ago


 Technology10 months ago
Technology10 months ago

 Technology10 months ago
Technology10 months ago