Health
Researchers have come close to making insulin pills
Published
1 year agoon

Australian researchers hope that a molecule different from insulin could one day help make insulin pills.People with type 1 diabetes cannot produce insulin and need multiple daily injections of insulin to control their blood glucose levels. This new research confirms that alternative molecules can be used to activate the absorption of blood glucose and bypass the need for insulin altogether.
Researchers at the “Walter and Eliza Hall Medical Institute” (WEHI) in Melbourne answered a 100-year-old question in the field of diabetes research. The question is whether a molecule different from insulin can have the same effect. These findings provide important insights for the development of an oral insulin pills in the future.
Insulin pills
This research group has shown how a molecule other than insulin can mimic the role of insulin, a key hormone for controlling blood sugar levels.
Walter and Eliza Hall Institute of Medicine research is paving new ways to develop drugs that could replace daily insulin injections for people with type 1 diabetes.
People with type 1 diabetes cannot produce insulin and need multiple daily injections of insulin to control their blood glucose levels. This new research confirms that alternative molecules can be used to activate the absorption of blood glucose and bypass the need for insulin altogether.
This research was conducted under the supervision of Dr Nicholas Kirk and Professor Mike Lawrence, researchers of the Walter and Eliza Hall Medical Institute, and in collaboration with the American pharmaceutical company Eli Lilly and Company. has been
Why are there no insulin tablets?

Scientists have tried to make insulin in pill form because insulin is unstable and is easily broken down by the body after it is digested, Dr Kirk said. Since the discovery of insulin about 100 years ago, making insulin pills has been a dream for diabetes researchers, but after decades of efforts, not much success has been achieved.
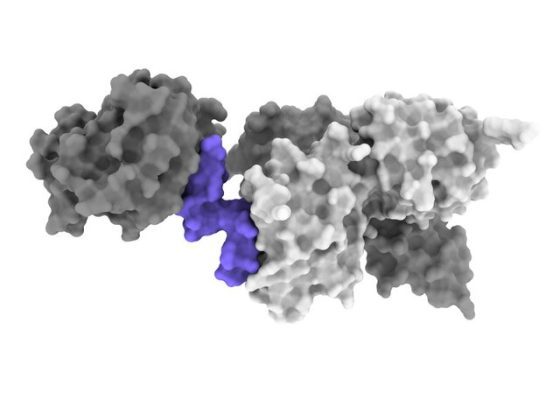
This research has now been dramatically accelerated by the development of cryo-electron microscopy (cryo-EM), which can visualize complex molecules in atomic detail, allowing researchers to rapidly produce 3D images of the insulin receptor.
Kirk continued: With cryo-electron microscopy, we can now directly understand how various molecules, including insulin, change the shape of the insulin receptor. Insulin interactions are much more complex than anticipated; so insulin and its receptors change dramatically.
New research shows how an insulin-mimicking molecule acts on the insulin receptor and turns it on. This is the first step in a pathway that directs cells to take up glucose when the body’s sugar levels are too high.
Read more: Why do we get sick in cold weather?
The research team performed complex cryo-electron microscopy reconstructions to obtain a map of several molecules called “peptides” that interact with the insulin receptor and hold it in an active position. Cryo-electron microscopy experiments identified a peptide that can bind to and activate the receptor like that of insulin.
“Insulin has evolved to hold the receptor as carefully as a hand holding a pair of pliers together,” Kirk said. The peptides we used work in pairs to activate the insulin receptor, like two hands holding a pair of pliers.
Although the time to reach therapeutic results is far away, the discovery of this research group can lead to the production of drugs to replace insulin and reduce the need for injections in patients with diabetes.
Scientists have succeeded in replacing these types of mimic molecules with drugs that can be taken as pills, Kirk said. Reaching this destination still has a long way to go, which requires more research, but it is interesting to know that our discovery paves the way for providing oral treatments for type 1 diabetes.
Via :Medicaldialogues


You may like

Many mental disorders have physical roots
Jessica Huston’s tics started when she was just 12 years old. Over time, his condition worsened until he had a seizure and was rushed to the hospital. Doctors at a local hospital in Durham, England, dismissed his condition and said he was suffering from anxiety and probably spent a lot of time watching TikTok videos.
Jessica actually suffers from an autoimmune disease caused by a streptococcal bacterial infection. His illness was a form of pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS). When the infection was identified and treated, her symptoms eventually began to improve.
Ms. Huston is not the only person with a brain dysfunction that is mistaken for a mental disorder. A lot of evidence shows that a series of infections can cause conditions such as obsessive-compulsive disorder, tic, anxiety, and even psychosis. Inflammatory and metabolic disorders can also have significant effects on mental health, although they are rarely considered by psychiatrists.
Rethinking the cause of mental disorders could have profound implications for the millions of people with mental illnesses who are currently undertreated. For example, more than 90% of patients with bipolar disorder develop recurrent illnesses during their lifetime. More than 46% of children with obsessive-compulsive disorder do not recover. About 50 to 60 percent of patients with depression eventually recover after trying different drugs. A deeper understanding of the biological components of mental health can lead to more accurate diagnoses and more targeted treatments.
Infections can cause obsessive disorder, tic, anxiety, and even psychosis
For a long time, the field of psychiatry has focused on describing and classifying symptoms rather than on underlying causes. The Diagnostic and Statistical Manual of Mental Disorders (DSM) was published in 1952 and contains descriptions, symptoms, and diagnostic criteria. Although this guide has helped unify diagnoses, it groups patients without considering the underlying mechanisms of mental disorders.
There is a lot of overlap between the symptoms of depression and anxiety, and some question whether they are really separate illnesses. At the same time, depression and anxiety exist in different forms. For example, panic disorder with and without agoraphobia are different diagnoses, but we may not find significant differences between them. This can lead to a high diversity of patients participating in drug trials, and these trials do not achieve results due to the few commonalities and large differences among the participants.
Previous attempts to find causal mechanisms for mental illness have been challenging. In 2013, the National Institute of Mental Health tried to distance itself from research based on classifications based on DSM symptoms. Huge budgets have been spent on research into brain disease processes with the hope of linking genes directly to behaviors. But this idea ultimately failed and most of the genes discovered had small effects.

Although genes may play a role in mental disorders, they are not the only answer. Many disorders such as schizophrenia, attention deficit hyperactivity disorder, anxiety, and autism can be caused by genetic disorder 22q11.2, in which part of chromosome 22 is deleted, says Ludger Tebartz van Elst, professor of psychiatry and psychotherapy at Freiburg University Hospital in Germany.
In 2007, studies conducted at the University of Pennsylvania showed that 100 patients with psychiatric symptoms or cognitive deficits actually had some kind of autoimmune disease. Their bodies were making antibodies against a key receptor in nerve cells called the NMDA receptor. This leads to swelling of the brain and can cause a wide range of symptoms including paranoia, hallucinations, and aggression. The disease described was called anti-NMDA receptor encephalitis, and in many cases, it was treatable by removing the antibodies or using immunotherapy drugs or steroids. Studies conducted on patients who had the first episode of psychosis have shown that between 5 and 10 percent of them also had antibodies that attack the brain.
It seems that in rare cases obsessive-compulsive disorder can also be caused by the immune system. This condition is seen in childhood PANDAS, which Ms. Houston was diagnosed within 2021. This disorder is sometimes seen in adults as well. A 64-year-old man spent a lot of time mowing his lawn, but the next day he felt remorse and guilt. The researchers found that these symptoms are caused by antibodies attacking the neurons in his brain.
Recently, Belinda Lennox, director of the Department of psychiatry at the University of Oxford, conducted experiments on thousands of patients with psychosis. He has found antibodies in blood samples of about 6% of patients, which mainly target NMDA receptors. He says it’s not clear how a set of antibodies can cause clinical symptoms ranging from seizures to psychosis to encephalitis. It’s also not clear why these antibodies are made or if they can cross the blood-brain barrier (the membrane that controls access to the brain). He hypothesizes that antibodies cross the blood-brain barrier and affect memory by binding to the hippocampus, leading to delusions and hallucinations.
Studies have shown that some patients with psychological symptoms or cognitive defects have some kind of autoimmune disease
Dr. Lennox says a medical rethink is needed to understand the damage the immune system can do to the brain. He is conducting experiments in this field.
Studies of patients with immune-mediated psychosis show that a wider range of strategies, including the removal of antibodies and the use of immunotherapy drugs or steroids, can be effective treatments.
People with myalgic encephalomyelitis/chronic fatigue syndrome (an infectious disease associated with a range of cognitive problems such as difficulty concentrating and paying attention) were once neglected or diagnosed as retarded. New research shows that myalgic encephalomyelitis is related to both immune disorders and metabolic disorders.
Metabolic disorders can also affect mental health. The brain is an extremely energy-demanding organ, and metabolic changes related to energy pathways are involved in various disorders, including schizophrenia, bipolar disorder, psychosis, eating disorders, and major depressive disorder.

At Stanford University, there is a metabolic psychiatry clinic where patients are treated with diet and lifestyle changes along with medication. An active area of research at this clinic is the potential benefits of a ketogenic diet, where carbohydrate intake is limited.
A ketogenic diet forces the body to burn fat for energy, creating chemicals known as ketones, which can be used as a fuel source in the brain when glucose is limited.
Metabolic disorders can affect mental health
Thirteen trials are underway around the world to examine the effects of metabolic therapies on serious mental illness, says Kirk Nylen, head of neuroscience at the US charity Bazoski Group, which funds brain research.
The preliminary results have shown that a large group of patients respond to these treatments in a meaningful way. Medicines, talk therapy, brain magnetic stimulation, and maybe electroshock therapy have not been effective for this group of patients.
It is not just the understanding of the immune and metabolic systems that is improving. Massive amounts of data are now being analyzed at unprecedented speed to reveal connections that were previously hidden from view. This could ultimately lead to more personalized and better treatments.
In early October 2023, the UK Biobank published data showing that people with depressive episodes had higher levels of inflammatory proteins such as cytokines in their blood. According to another study, about a quarter of depressed patients showed evidence of mild inflammation. Knowing this can be helpful because other research shows that patients with inflammation respond poorly to antidepressants.
There are new advances in understanding the underlying causes of mental disorders. A group of researchers are investigating different ways to improve the diagnosis of ADHD; Like classifying patients into different subgroups, some of which were previously unknown. Different groups of researchers announced in three different statements in February 2024 the discovery of biomarkers that can predict the risk of dementia, autism, and psychosis.
The search for better diagnostic tools is also likely to be accelerated by the use of artificial intelligence. A company called Cognoa is using artificial intelligence to diagnose autism in children by analyzing videos of their movement behaviors in doctors’ waiting rooms.
The Quantitative Biosciences Institute (QBI) in California has used artificial intelligence to create an entirely new map of the interactions between proteins and molecular networks involved in autism. This will greatly facilitate finding diagnostic and therapeutic tools.
The developments mentioned are promising. But many problems can be solved by reducing the gap that exists today between neurology and psychiatry. Neurology studies and treats physical, structural, and functional disorders of the brain, while psychiatry deals with mental, emotional, and behavioral disorders. Dr. Lennox envisions a future in which antibody testing is performed when a person who develops a sudden mental breakdown after a viral infection fails to recover with standard treatments.
According to Dr. Tebartz van Elst, the gap between neurology and psychiatry is greater in Anglo-Saxon countries (including the United States, Great Britain, Canada, and New Zealand). In Germany, psychiatry and neurology are closer together, so neurologists are trained in psychiatry and psychiatrists are trained in neurology for one year. This makes research work easier.
For most patients who are first diagnosed with psychosis or other severe psychiatric syndromes, Dr. Tebartz van Elst prescribes brain MRIs, electroencephalograms, laboratory tests for inflammation, and lumbar punctures in order to better treat them by finding clues to the cause of the illness. Submitted.


Why do people listen to sad songs?
There is a paradox in sad music: we don’t enjoy sadness in real life, but we enjoy art that makes us feel that way. Countless researchers since Aristotle tried to solve this contradiction. Perhaps through music, we experience a kind of catharsis of negative emotions. Catharsis here means refinement and cultivation of the soul. Maybe there is an evolutionary advantage in this feeling of sadness, or maybe we want to value our suffering. Maybe our body produces hormones in response to anxiety disorder, music that leads to a sense of comfort.
According to the New York Times, Dr. Nob, an experimental philosopher and psychologist at Yale University, in a new study published in the Aesthetic Education Journal, raised the question, “What is the purpose of sad music?” He tried to solve the contradiction of this kind of music. Over the years, he came to the conclusion that people often have two perceptions of the same thing. For example, they can consider people as artists if they have a set of characteristics such as an innate talent for working with a brush; But if they don’t have abstract values such as creativity, curiosity or interest and just recreate old masterpieces for profit, we can say they are not artists. According to Dr. Nob and his former student Tara Venkatsan, a cognitive scientist, perhaps sad music also has a dual nature.

The aforementioned research shows that our emotional response to music is multidimensional; You don’t necessarily feel happy when you listen to a beautiful song, and you don’t necessarily feel sad when you listen to a sad song. According to a 2016 study, the emotional response of 363 listeners to sad songs was divided into three categories: sadness and strong negative emotions such as anger, panic, and despair, nostalgia, quiet sadness and self-compassion, and finally sweet sadness is pleasant pain. It comes from consolation and understanding. Many respondents reported a combination of all three. The researchers called this research “Fifty Blue Spectrums”.
Given the layers of emotion and the ambiguity of language, it’s no wonder that sad music creates a paradox; But it is not clear why it induces a sense of pleasure or meaning. Some psychologists have investigated how certain aspects of music, such as position, pitch, rhythm, and resonance, are related to listeners’ emotions. According to research, certain forms of songs have an almost universal function: for example, among different countries and cultures, lullabies have similar acoustic characteristics that make children and adults feel safe. Thomas Irola, a musicologist at the University of Durham in England and researcher of the “Fifty Spectrum” study, says:
Throughout life, we learn to make connections between our feelings and what we hear. We recognize emotional expression in speech, and often these cues are used in a similar way in music.
Other researchers, such as Patrick Joslin, a music psychologist from Uppsala University in Sweden, believe that such findings reveal the value of sad music. Sad music, he writes in an essay, asks why “the second movement of Beethoven’s Eroica symphony evokes a sense of sadness?” It leads to the question, “Why does a slow step lead to a feeling of sadness?”
According to the findings of Joslin and his colleagues, there are cognitive mechanisms through which feelings of sadness are induced in listeners. These mechanisms include unconscious reactions in the brain stem, synchronization of the rhythm with the internal rhythm such as the heartbeat, conditional reactions to certain sounds, the arousal of memories, emotional contagion, and reflexive measurement of music. Perhaps because sadness is such a strong emotion, it can evoke an empathetic and positive response. In fact, understanding other people’s grief provokes a social response.
Why do people listen to sad songs?
The purpose of listening to sad music is not necessarily to convey sadness; Rather, it is creating a sense of connection.
Dr. Nob, along with Dr. Venkatsan and George Newman, a psychologist at the Rotman School of Management, designed a two-stage experiment to test the hypothesis. In the first part of the experiment, they gave one of four song descriptions to more than 400 participants. In the description of the first song, it was written: “transmitting complex and deep emotions, but technically full of errors.” The second track was described as: “music without technical errors that do not convey complex and deep emotions.” The third song was described as “highly emotional and technically flawless” and the fourth song was described as “technically flawed and non-emotional”.

Subjects were asked to indicate on a seven-point scale whether their song conveyed the intent of the music or not. Their goal was to show how important it is for music to express emotion and generally happiness, sadness, hate, or any other emotion on an intuitive level. Overall, subjects reported that deeply emotional but technically flawed songs best reflected the nature of music. In other words, the emotional expression had a more prominent value than the technical aspect.
In the second part of the experiment, which included 450 new subjects, the researchers gave each participant 72 descriptions of emotional songs that convey feelings such as “humiliation,” “narcissism,” “inspiration,” and “lust.” For comparison, they gave participants phrases that convey conversational interaction in expressing people’s feelings. For example, one of the phrases was: “An acquaintance is talking to you about the past week and his feeling of passion”. In general, the emotions that the subjects receive are strongly rooted in the “purpose of the music” and are similar to the emotions that make people feel close to each other in conversation: emotions such as love, joy, loneliness, sadness, ecstasy, and relaxation.
Mario Etti Picker, a philosopher at Lowell University of Chicago, finds the results of this study interesting. After reviewing the data, he came up with a relatively simple idea: “Perhaps the reason we listen to music is not just an emotional response, but we do it to understand the connection with others; Because, according to the reports of many subjects, sad music is not necessarily enjoyable despite its artistic dimensions. In other words, according to the paradox of sad music, our love of music is not the result of direct praise of sadness; Rather, it is the result of valuing communication with others.”
Dr. Irola also concluded in his research that empathic people are likely to be moved by unfamiliar sad music. They tend to engage in this kind of imaginary grief. These people also show significant hormonal changes in response to sad music. But sad music, like an onion, has many layers, and this explanation can give rise to other questions. For example, who should we communicate with? Artist or with our own past? Or even with an imaginary person?

A new drug developed to treat severe influenza works in a unique way, unlike what a drug would expect, to treat lung disease and infection.
Inventing a new drug to treat influenza!
A new drug to treat severe flu successfully keeps patients at the right level of lung inflammation to protect against lung damage while still allowing the immune system to fight infection. This drug has been effective in mice even a few days after infection.
According to New Atlas, if you’ve ever had the flu, you’ve most likely contracted the influenza A virus (IAV). Compared to influenza B virus, infection with type A often causes more severe symptoms. But, while many of us have experienced the fever and chills, headache and muscle aches, fatigue, sore throat, and cough of the common flu, severe infection with the animal IAV strain is different and potentially life-threatening.
Severe infection of this type of influenza causes a special type of cell death called necroptosis in infected cells. While this is a natural process designed to limit viral spread by actively eliminating infected cells and mobilizing the immune system to respond, necroptosis can activate a hyperinflammatory response and cause collateral lung damage that is potentially fatal. Is. Other than managing its symptoms, there are few treatment options for treating severe influenza.
In a new study, researchers from Tufts University School of Medicine, St. Jude Children’s Research Hospital, the University of Houston, and Fox Chase Cancer Center collaborated to test a drug called UH15-38 that could prevent this flu-related lung damage in mice. It prevents and allows the immune system to fight the virus.
“Our drug significantly increased survival and reduced symptoms of influenza virus infection,” said Paul Thomas, co-author of the study. The new drug reduced dangerous inflammation and even seemed to improve the adaptive response to the virus.
Achieving the Goldilocks effect, or the effect of the right amount of the drug on inflammation, required researchers to use clever chemistry along with a thorough understanding of the underlying mechanisms of necroptosis.
Receptor-interacting protein kinase 3 (RIPK3) is an essential part of the necroptosis cell death pathway, but it also controls another cell death pathway called apoptosis. Both types of cell death trigger opposing immune responses. Apoptotic death usually results in muted immunological responses, while necroptosis releases molecules that cause inflammation. UH15-38 was designed to prevent the stimulation of the necroptosis pathway by RIPK3, while still allowing cell death and removing infected cells in a less inflammatory manner.
Alexei Degterev, an associate professor of developmental, molecular, and chemical biology at Tufts University School of Medicine and one of the authors of the paper, says: “If you eliminate necroptosis, you will still limit virus replication without severe damage to the lungs.” Necroptosis does not appear to be necessary to limit viral activity, so if we can block it, we can protect the host by reducing inflammation in the lungs.
Read more: Testing a vaccine that reduces liver tumors
The researchers tested the drug UH15-38 in mouse models and found that high doses of the drug provided protection against the usually fatal IAV influenza. At low doses, the UH15-38 drug protected mice against similar amounts of influenza that humans experience. Notably, the mice were protected even if they received the drug several days after being infected with the disease.
“This drug can do something we haven’t seen before,” says Thomas. We can start five days after the initial infection and still see benefits. Completely removing the RIPK3 protein is not a great choice because then the immune system cannot clear the virus. When we removed only the necroptosis, the animals did better because they still had apoptosis and could still get rid of the infectious cells, but their condition was not as severely inflammatory.
UH15-38 improved survival by preventing collateral necroptosis damage to type 1 alveolar epithelial cells, a special type of cell in the lungs that facilitates gas exchange. Damage to these cells can make it difficult for oxygen to enter the blood and carbon dioxide from it, and cause symptoms such as shortness of breath, wheezing, and chest tightness. The drug also reduced the number of immune cells associated with inflammation, such as neutrophils, in the mice.
Often, the worst part of the flu happens after the virus is under control when inflammation destroys lung cells, Thomas says. UH15-38 can reduce influenza-induced inflammation while leaving viral clearance and other functions of tissue and immune responses intact. This makes the drug a promising option to move towards clinical use.
The next step is clinical and human trials safely. Researchers are testing whether UH15-38 is effective in treating other respiratory diseases.
While the worst of COVID-19 may be upon us, another pandemic is expected, and we need something that protects the host regardless of how it is infected, Degtref says. This study demonstrates the possibility of achieving such a goal and renews interest in how cell death occurs against infections.
This study was published in the journal Nature.


James Webb space telescope map of the climate of an exoplanet


The highest observatory in the world officially started its work


Many mental disorders have physical roots


How to connect to the TV with a Samsung phone?


What is an exoplanet? Everything you need to know


The secret of the cleanest air on earth has been discovered


Asus Zenbook 14 OLED laptop review


Motorola Edge 50 Pro


How extinct animals could be brought back from death?


Healing diabetic wounds with bacteria-killing hydrogel
Popular
-



 Technology9 months ago
Technology9 months agoWho has checked our Whatsapp profile viewed my Whatsapp August 2023
-



 Technology10 months ago
Technology10 months agoHow to use ChatGPT on Android and iOS
-



 Technology9 months ago
Technology9 months agoSecond WhatsApp , how to install and download dual WhatsApp August 2023
-



 Technology11 months ago
Technology11 months agoThe best Android tablets 2023, buying guide
-


 Humans1 year ago
Humans1 year agoCell Rover analyzes the inside of cells without destroying them
-



 AI1 year ago
AI1 year agoUber replaces human drivers with robots
-



 Technology10 months ago
Technology10 months agoThe best photography cameras 2023, buying guide and price
-


 Technology11 months ago
Technology11 months agoHow to prevent automatic download of applications on Samsung phones






