Health
Dark matter secrets of the human genome
Published
5 months agoon

Dark matter secrets of the human genome
In this article, we’re going to read about the dark matter secrets of the human genome. Twenty years ago, a massive scientific effort showed that the human genome contains 20,000 protein-coding genes, but they make up only 2% of our DNA. The rest of the genome was considered useless or redundant DNA, but we now realize it plays an important role.
When it was announced in April 2003 that the 13-year effort to sequence the entire book of life encoded within the human genome was complete, there were high expectations. There was hope that the $3 billion Human Genome Project would lead to cures for chronic diseases and provide insights into everything in our lives that is determined by genetics.
But even as press conferences were being held to announce this achievement, this guide to human life surprised scientists.
At the time, the prevailing belief was that most of the human genome consisted of instructions for building proteins, the building blocks of living organisms, which play various roles within and between our cells, writes the BBC.
With more than 200 different types of cells in the human body, it seemed logical that each would have its own set of genes to perform its essential functions. The emergence of unique sets of proteins was thought to be critical to the evolution of our species and our cognitive powers.
Instead, it turns out that less than two percent of the three billion letters in the human genome are devoted to proteins. Only about 20,000 distinct protein-coding genes were found in the long string of molecules known as base pairs that make up our DNA sequences.
Geneticists were surprised to find that the number of protein-making genes in humans is similar to that of some of the simplest organisms on Earth. Suddenly, the world of science was faced with the unpleasant reality that perhaps most of our understanding of what makes us human has been wrong.
Humans have a total of about 19,000 protein-making genes, while worms have about 20,000, and fruit flies have about 13,000 protein-making genes.
“I remember that unbelievable shock,” says Samir Onezin, a molecular biologist and CEO of Haya Therapeutics, which is trying to use insights from studying human genetics to develop new treatments for cardiovascular disease, cancer, and other chronic diseases. “At that moment, people began to think that maybe our understanding of biology was wrong.”
The remaining 98 percent of our DNA is known as “dark matter” or the “dark genome.” At first, some geneticists proposed that the dark genome was the junk DNA or garbage dump of human evolution and the remnants of defective genes that had long since lost their significance.
Although it was obvious to others that the dark genome was vital to our understanding of being human. “Evolution has absolutely no tolerance for junk,” says Kari Stefansson, CEO of the Icelandic company that decodes genetics, which has sequenced more of the entire human genome than any other institution in the world. “There must be an evolutionary reason to maintain this genome size.”
Read More: Scientists discovered the secret of DNA’s X shape
Dark matter secrets of the human genome
Now, two decades later, we have our first insights into the role of the dark genome. It seems that the main function of the dark genome is to regulate the decoding process or the expression of protein-making genes. This part of the genome helps control how our genes behave in response to all the environmental pressures our bodies face throughout our lives, from diet and stress to pollution, exercise, and sleep.


Onezin sees proteins as the hardware components of life, while the dark genome is software that processes and responds to external information. As a result, the more we learn about the dark genome, the more we understand the complexity of humans and how we become human. “If we think of ourselves as a species, we see that we are adaptive to the environment at every level,” says Onezin. This adaptation is information processing. “When you go back to the question of what makes us different from a fly or a worm, we find that the answers lie in the dark genome.”
Dark matter secrets of the human genome
Transposons and our evolutionary past
When scientists first began poring over the Book of Life in the mid-2000s, one of the biggest challenges was that the non-protein-coding regions of the human genome appeared to be full of repetitive DNA sequences called transposons. These repetitive sequences are so abundant that they make up almost half of the entire genome of all living mammals. “Even reconstructing the first human genome was more difficult with these repetitive sequences,” says Jeff Boke, who directs the Dark Matter Project at New York University’s Langone Medical Center. “If the sequence is unique, it’s easier to analyze.”
At first, geneticists ignored transposons. Most genetic studies have focused solely on the exome (the small protein-coding region in the genome). But in the past decade, the advent of advanced DNA sequencing technologies has allowed geneticists to study the dark genome in greater detail.
An experiment in which researchers deleted a specific transposon fragment in mice, causing half the pups to die before birth, showed that some transposon sequences may be critical to our survival.
Perhaps the best explanation for why transposons are present in our genomes is that they are very old, dating back to the earliest life forms, Bocke says. Other scientists have suggested that transposons come from viruses that invaded our DNA throughout human history and then gradually repurposed in the body to find useful targets.
“In most cases, transposons are pathogens that infect us, and they can infect germline cells, the cells that we pass on to the next generation,” says Dirk Hockmeyer, assistant professor of cell biology at the University of California, Berkeley. “They can then be inherited and permanently integrated into the genome.” Bokeh describes the dark genome as something that acts like a living fossil record of vital changes in our DNA that occurred long ago in ancient history.
One of the most interesting properties of transposons is that they can jump from one part of the genome to another and cause mutations that sometimes have significant consequences.
The movement of a transposon to a different gene may have caused the loss of the tail in the family of large copies, which led to our species gaining the ability to walk upright. “This unique event had a great impact on evolution and gave rise to a lineage of large replicas, including humans,” says Boke.
But as our growing understanding of the dark genome continues to shed more light on evolution, the dark genome could also provide insights into how diseases arise.
Onezin points out that if you look at genome-wide association studies (GWAS), most of the genetic sequences associated with chronic diseases such as Alzheimer’s, diabetes, and heart disease are not in protein-coding regions, but in the dark genome. Genome-wide association studies examine genetic variation in large numbers of individuals to identify genetic variants associated with diseases.
Dark matter secrets of the human genome
The dark genome and diseases
Panay Island in the Philippines is famous for its sparkling white sands and constant influx of tourists, but this amazing place hides a sad secret.
This island has the highest frequency of incurable movement disorder called X-linked parkinsonism dystonia, abbreviated as XDP. Like Parkinson’s disease, people with XDP experience a range of symptoms that affect the ability to walk as well as the ability to react quickly to different situations.
Since XDP was first discovered in the 1970s, the disorder has so far only been seen in people of Filipino descent. The reason for this was a mystery for a long time until geneticists realized that all these people had a unique variant of a gene called TAF1.
The onset of symptoms appears to be driven by a transposon in the middle of this gene, which can alter its function in ways that damage the body over time. This gene variant is thought to have appeared for the first time about two thousand years ago and then became fixed in the population. “The TAF1 gene is an essential gene, meaning that it is needed for the growth and reproduction of all types of cells,” Boke says. “When you change its expression, you end up with this very specific defect that appears in this horrible form of parkinsonism.”
The above case is a simple example of why some DNA sequences in the dark genome can control the function of different genes or activate or suppress the process of converting genetic information into protein in response to environmental signals.
The dark genome also carries instructions for making different types of molecules known as non-coding RNAs. Non-coding RNA molecules have different roles such as helping to form proteins, inhibiting the protein production process, or helping to regulate gene activity. “The RNAs produced by the dark genome act like conductors, directing how the DNA responds to the environment,” Onezin says.
Non-coding RNA is now increasingly considered as a link between the dark genome and various chronic diseases.
The common thinking is that if we continuously give the dark genome the wrong signal (eg, through a smoking lifestyle, poor diet, and inactivity), the RNA molecules that are produced can put the body into a disease state and alter gene activity in some way. which increases inflammation in the body or causes cell death.
Some non-coding RNAs are thought to affect the activity of a gene called p53, which normally acts to prevent the formation of tumors. In complex diseases such as schizophrenia or depression, the unfavorable set of non-coding genes may act in concert to decrease or increase the expression of some genes.
Our growing understanding of the importance of the dark genome has now led to new approaches to treat these diseases. While the pharmaceutical industry usually focuses on proteins, some believe that trying to disrupt the non-coding RNAs that control the genes responsible for these processes may be a more effective approach.
In the field of cancer vaccines, where companies genetically sequence a patient’s tumor sample to identify a suitable target for the immune system to attack, most approaches have focused only on protein-coding regions. However, the German pharmaceutical company Curroc is pioneering an approach in which it also analyzes non-coding regions of proteins in the hope of finding a target that can disrupt the source of cancer.
Onezin’s company, Haya Therapeutics, is pursuing a drug development program that targets a set of non-coding RNAs that cause scar tissue, or fibrosis, in the heart. The formation of scar tissue in the heart can lead to heart failure.
Researchers hope that this approach can minimize the side effects of many common drugs. “The problem with protein-based medicine is that there are only about 20,000 proteins in the body, and most of them are expressed in different cells and in pathways unrelated to disease,” Onezin says. However, the dark genome is very specific in its activity. “There are non-coding RNAs that regulate fibrosis only in the heart, so with a drug based on those, you might get a very safe drug.”
Dark matter secrets of the human genome
Unknowns
We know very little about what geneticists describe as ground rules: how these non-coding sequences interact to regulate gene activity. And how do these complex chains of interactions manifest themselves over time in the form of disease and, for example, cause the neurodegeneration seen in Alzheimer’s disease?


“Right now, we’re at the beginning of the journey,” Hockmeyer says. The next 15 to 20 years will be important. In the coming years, researchers will work on identifying specific behaviors in cells that can lead to disease, as well as identifying parts of the dark genome that can play a role in modifying these behaviors. “We now have tools to explore this that we didn’t have access to before.”
One of these tools is gene editing. By cloning the TAF1 gene transposon in the mouse genome, Boke and his team are trying to learn more about how XDP symptoms develop. In the future, a more ambitious version of the project could try to find out how non-coding DNA sequences regulate genes by making pieces of DNA and transferring them to mouse cells.
“We’re currently working on two projects where we’re taking a large piece of non-functional DNA and then trying to insert these elements into it,” says Boke. We insert a gene into that sequence, then we insert a neutralizing sequence right in front of it and another sequence further away from it, and we study how the gene behaves. “We now have the tools to make pieces of the dark genome and study and understand it.”
Hackmeier predicts that as we gain more knowledge, just like when the first genome was sequenced 20 years ago, the Book of Life will continue to yield unexpected surprises. “There are so many questions,” he says: “Is our genome still evolving?” Can we fully decipher it? “We are still in this unknown space and we are exploring it and there are some very interesting discoveries that we can make.”


You may like
-




Discovery of the brain circuit that manages inflammation
-


Skin cancer: symptoms, prevention and treatment
-


The strange ways skin affects our health
-



Artificial intelligence identifies cancer killer cells
-




Discovery of 32 new cancer drugs with the help of artificial intelligence
-


Brain cancer vaccine success in human trials

Researchers believe that using this new brain circuit could lead to new treatments for many immune disorders.
Discovery of the brain super circuit that manages inflammation
Researchers have found that brainstem neurons act as regulators of inflammation. These neurons can increase or decrease inflammation in response to signals sent by the vagus nerve, a collection of thousands of nerve fibers that connect the brain and internal organs.
A new study in mice shows that a peripheral immune stimulus powerfully activates the body-brain axis to regulate immune responses, according to AI. Pro- and anti-inflammatory cytokines communicate with specific populations of vagal neurons to inform the brain of an emerging inflammatory response. The brain, in turn, strongly modulates this environmental immune response process.
Cytokines are a group of water-soluble protein molecules that are secreted from various cells in response to a stimulus and are responsible for transmitting messages between cells. The consequence of the presence of cytokines is a change in the behavior of cells with secreted cytokine receptors, including growth, change, or cell death. The action and effect of cytokine produced by one cell includes more cells around the same cell, but it can have a systemic action and effect on the whole organism.
Cytokine has the effect of changing the secreting cell itself and changes in other cells, and like a hormone, it can have effects on cells far away from it.
The vagus nerve is also the longest brain nerve and the tenth pair of brain nerves out of 12 pairs of brain nerves, which is involved in swallowing food, speaking, parasympathetic activities, and digestion. The motor part of this nerve is somatic and innervates the larynx, soft palate, and pharynx. This nerve is the longest cranial nerve, and like most cranial nerves, it starts from the brain stem and is divided into many branches that innervate most of the muscles of the pharynx and larynx, esophagus, stomach, and parasympathetic heart, lung, liver, spleen, etc.
Discovery of the neuro-immune axis
Based on this study, the researchers used single-cell RNA sequencing, combined with functional imaging, to identify circuit components of this neuro-immune axis and show that its selective manipulation can effectively suppress the pro-inflammatory response while maintaining an anti-inflammatory state.
This new brain circuit, like a thermostat, helps increase or decrease inflammatory responses so the body responds in a healthy way, said Dr. Hao Jin, who began the study as a postdoctoral researcher in Dr. Zucker’s lab.
Looking at past research, it makes sense that a master regulator controls this critical response, the researchers say. Many psychosomatic effects can actually be related to brain circuits that tell your body something.
They believe that using this new brain circuit could lead to new treatments for many immune disorders.
Promising therapeutic potential
Brain-induced transformation of an immune response pathway offers new possibilities in modulating a wide range of immune disorders, from autoimmune diseases to cytokine shock.
“This new discovery could open up an exciting therapeutic area for controlling inflammation and immunity,” said Charles Zucker, senior author of the study.
Researchers believe that controlling this newly discovered brain circuit could lead to new treatments for common autoimmune diseases including rheumatoid arthritis, type 1 diabetes, multiple sclerosis, lupus, and inflammatory bowel disease.
Read more: Brain cancer vaccine success in human trials
This new control agent could also help treat other diseases such as prolonged COVID-19 syndrome, organ transplant rejection, and cytokine storms caused by COVID-19. According to the researchers, inhibiting the activity of this circuit could make a difference in a wide range of conditions that affect the immune system and help treat dysregulated inflammatory states in people suffering from diseases and immune disorders. This study was published in the journal Nature.
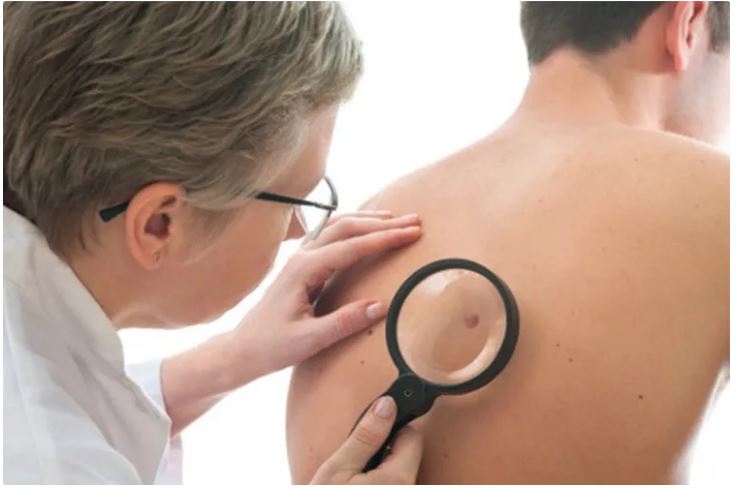

Skin cancer: symptoms, prevention and treatment
Skin cancer is the abnormal growth of skin cells. This problem generally occurs in areas of the skin that are exposed to sunlight, but sometimes it occurs in areas of the skin that are not normally exposed to light.
Brief anatomy of the skin
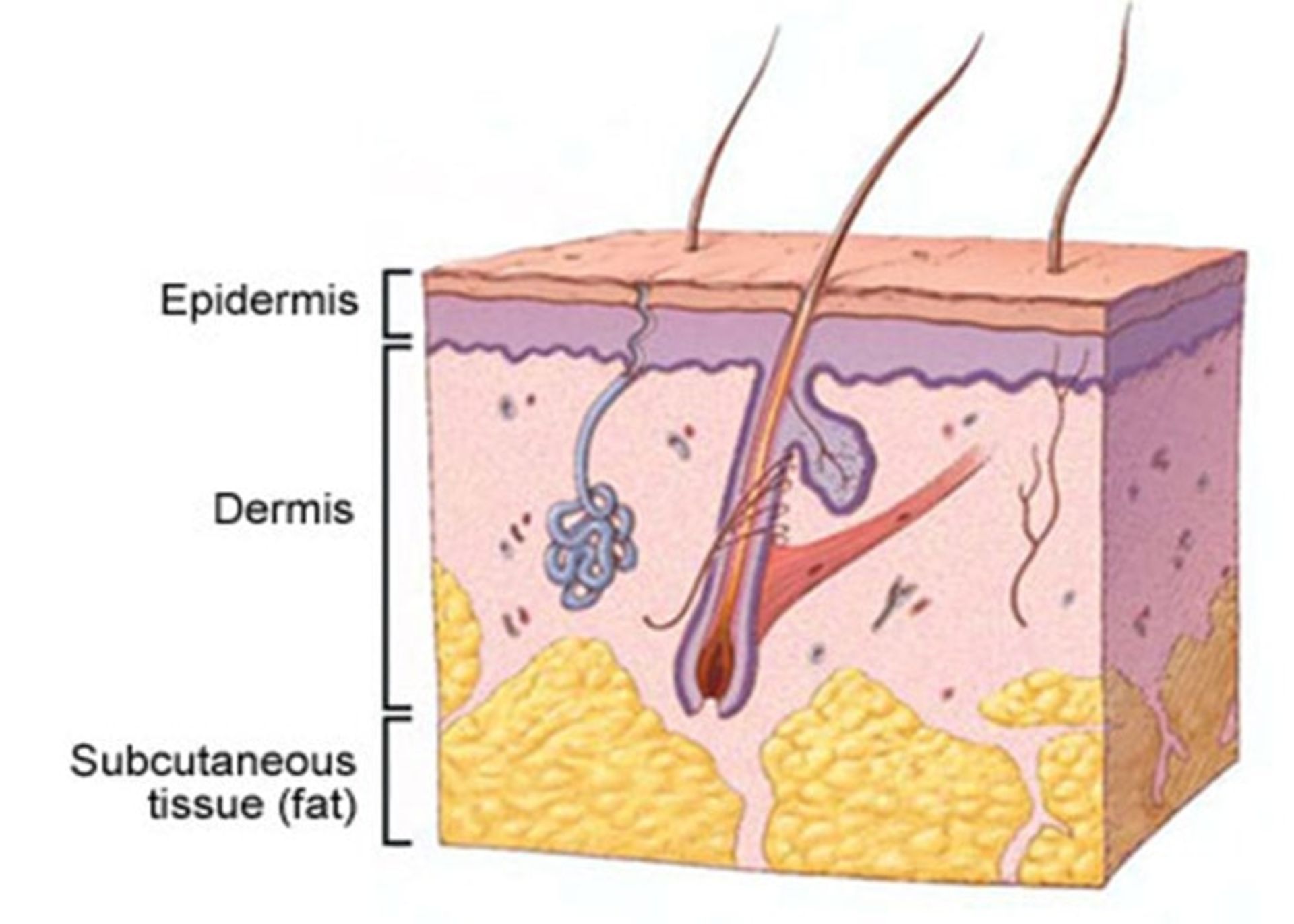
The skin is the largest organ of the body and consists of three main layers:
- Epidermis: It is a very thin outer layer of the skin that acts as a barrier to environmental infections and controls the amount of water that evaporates from the body.
- Dermis: The middle and thicker layer of the skin that contains hair follicles as well as nerves and a large number of blood and lymph vessels embedded in a framework of collagen.
- Hypodermis: the deepest layer of the skin, which is rich in fat and collagen and has many blood and lymphatic vessels.
Most skin cancers appear from the cells in the epidermis layer. Keratinocytes are the main cells in this layer of the skin. Deep in the epidermis and near the dermis layer, these cells grow and actively produce new skin cells. This layer is the basal cell layer. Over time, these cells migrate to the surface of the skin and become squamous cells. Among the basal cells, there are brown pigments called melanin that make up the melanocyte cells. These are located close to other cells that remove foreign substances such as bacteria or cancer cells from the skin and deliver them to the lymph nodes through the lymphatic vessels (ie Langerhans cells of the skin).
The lymphatic vessels of the skin reach the lymph nodes of the groin, armpit, and neck. These vessels carry lymph fluid and are one of the fluid circulation systems in the body. This system is considered part of the body’s immune system, but at the same time, it is a pathway that skin cancer cells can use to spread throughout the body. Lymph nodes act as a filter and trap these cells. The proliferation of these cells in the lymph nodes causes the lymph nodes to enlarge.
Types of skin cancer
There are two types of skin cancer based on the type of cells involved:
Keratinocyte cancer
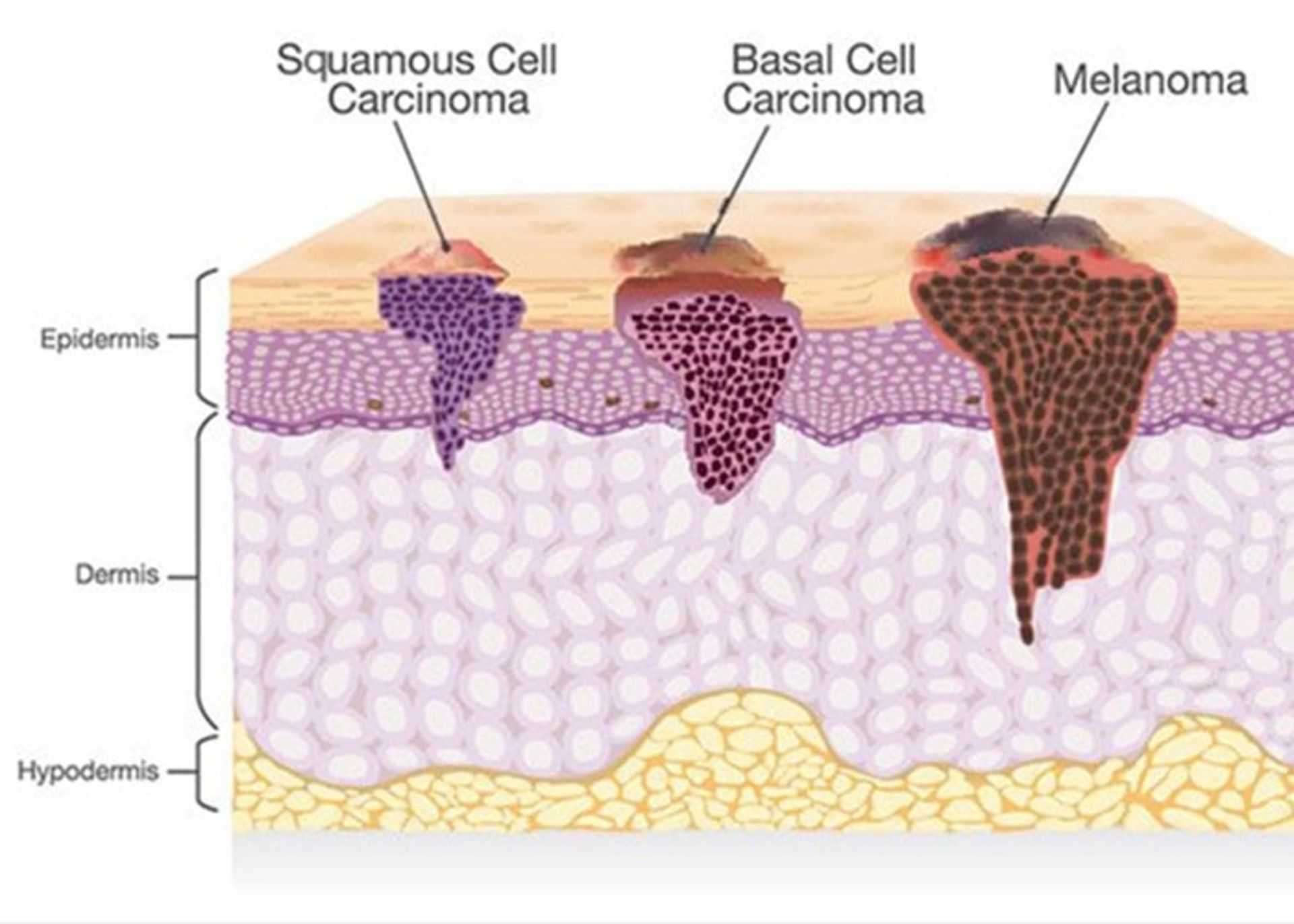
This type of skin cancer includes basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). These are the most common forms of skin cancer. These are more likely to develop in areas of the body that are exposed to sunlight more, i.e. the head and neck. In this form of skin cancer, the probability of the spread of cancer cells and the death of the patient is less than in other types, but if it is not treated, it may grow and spread to other parts of the body.
Squamous cell cancer: This type of cancer occurs in the outer layer of the skin and is usually more aggressive than basal cell cancer. This disease may appear as red and scaly lesions on the skin.
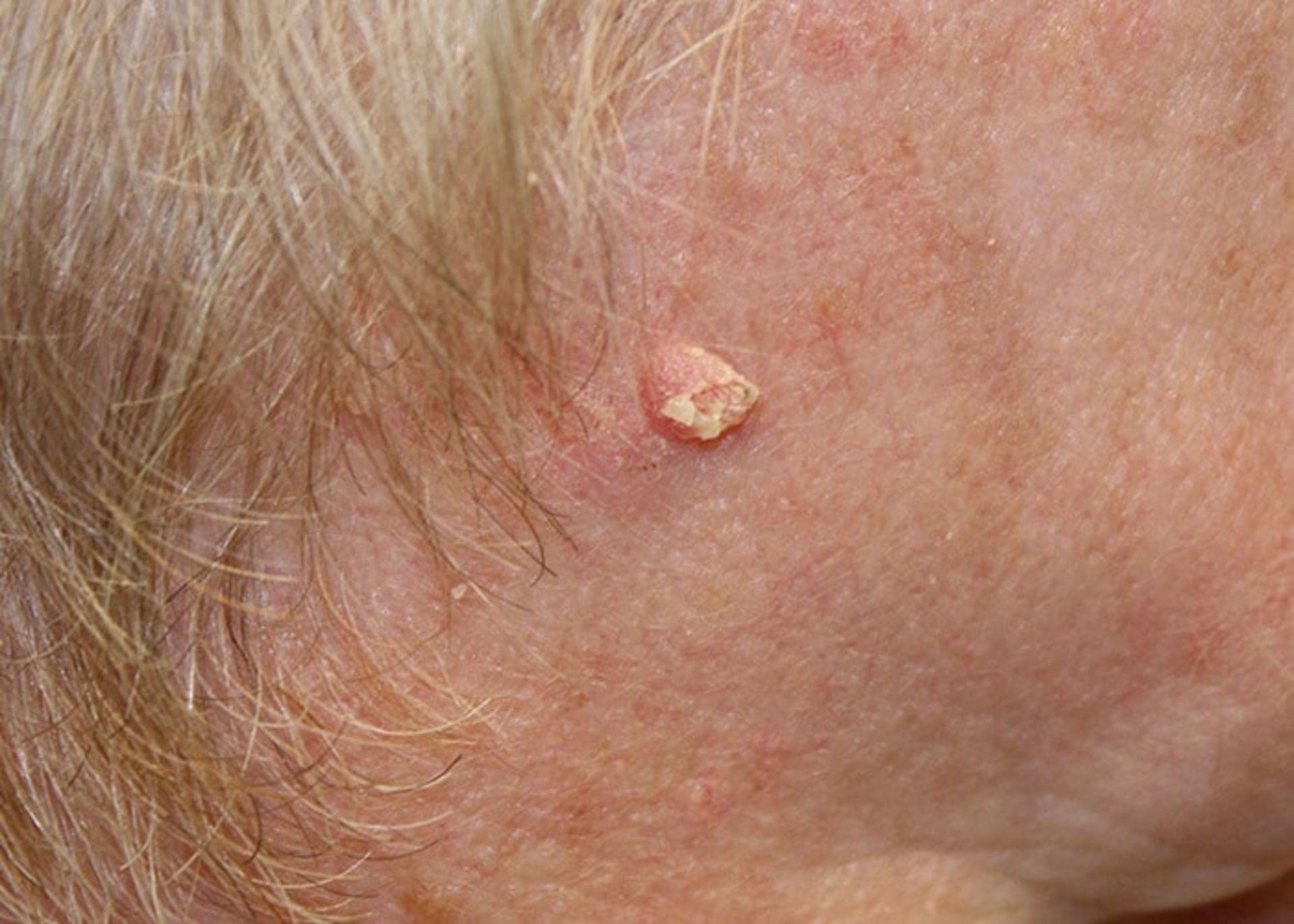
Squamous cell skin cancer
Basal cell cancer: The most common form of skin cancer is basal cell cancer, which accounts for 90% of all skin cancer cases. These are slow-growing lumps that often appear in the head and neck area.
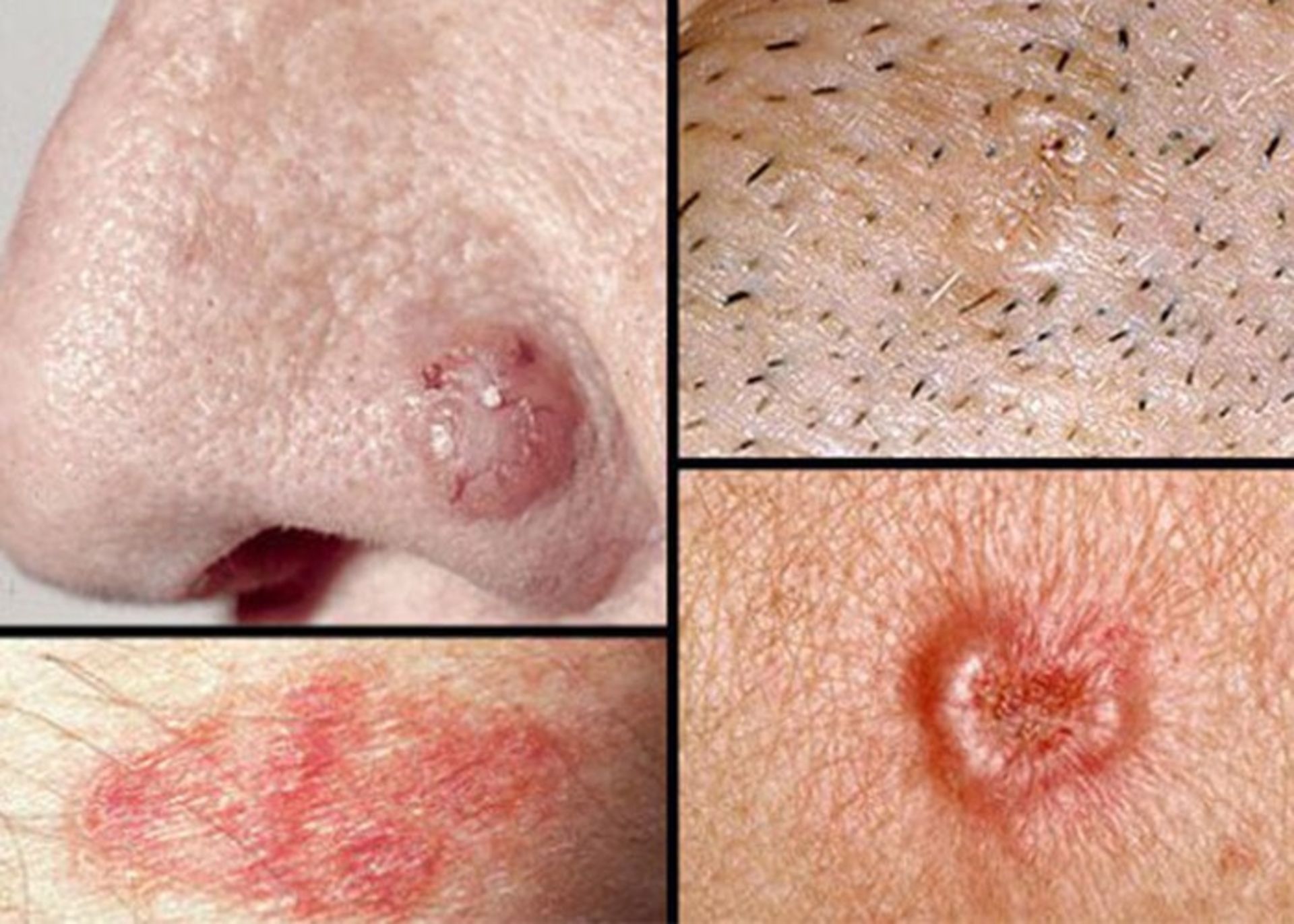
Basal cell skin cancer
Melanoma cancer
The second type of skin cancer is melanoma. This type of cancer is related to melanocytes. Benign moles created by melanocytes can become cancerous. These moles can form anywhere on the body, but they are more likely to develop on the chest and back. In women, the possibility of forming these moles on the legs is more. Most melanomas are curable if diagnosed and treated promptly. If left untreated, they can spread to other areas of the body and become more difficult to treat. This type of skin cancer is more likely to spread than basal cell cancer and squamous cell cancer. This type of skin cancer is less common, but it is considered the most dangerous type of skin cancer. In fact, melanoma only accounts for one percent of skin cancers, but the majority of skin cancer-related deaths each year are related to this disease.
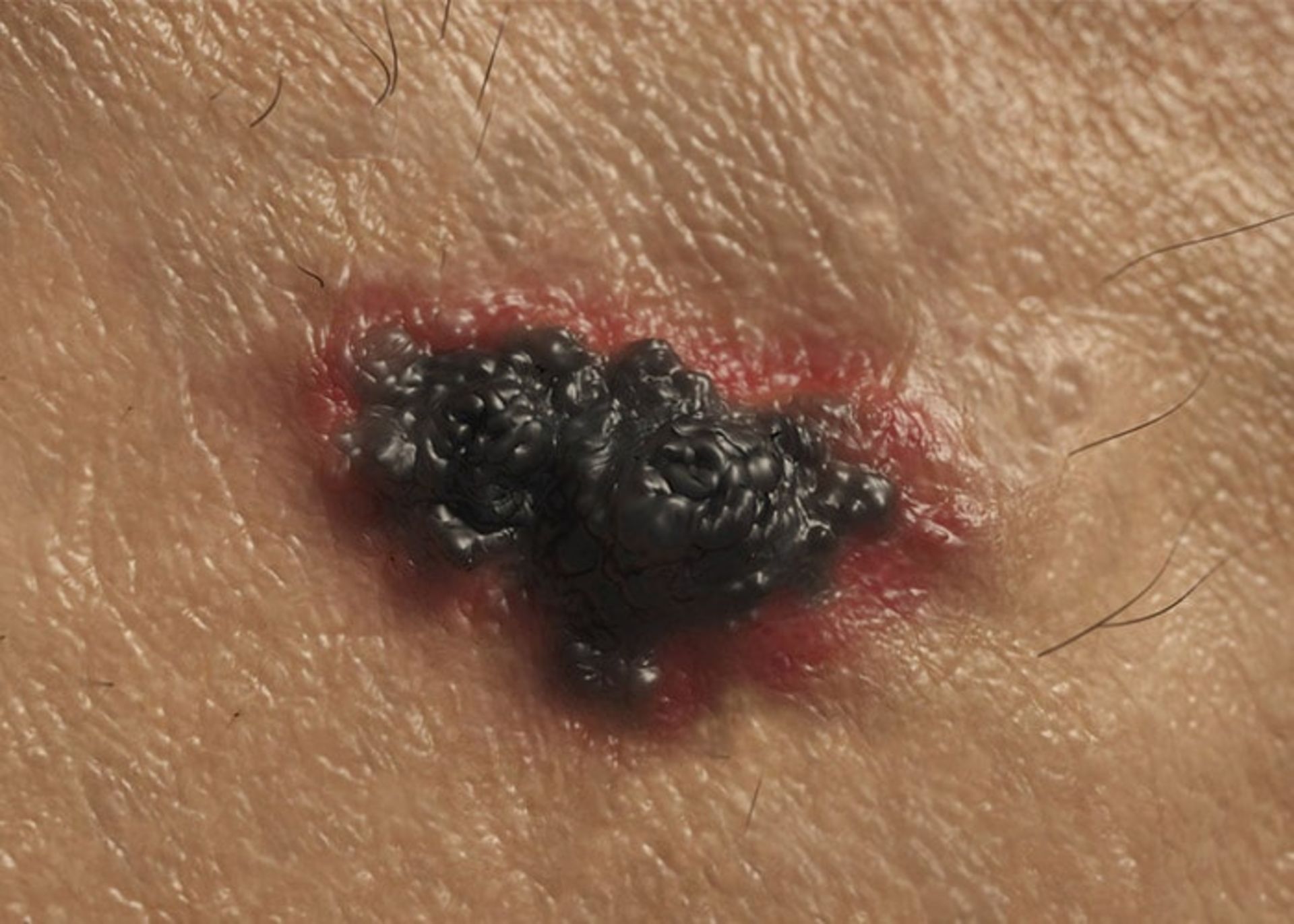
Melanoma cancer
There are types of skin lesions that are a subset of skin cancer. All of these are not skin cancers, but they can become cancerous. One of these cases is actinic keratosis: these red or pink lesions on the skin are not cancerous but are considered a precancerous condition. If left untreated, it can turn into squamous cell carcinoma.
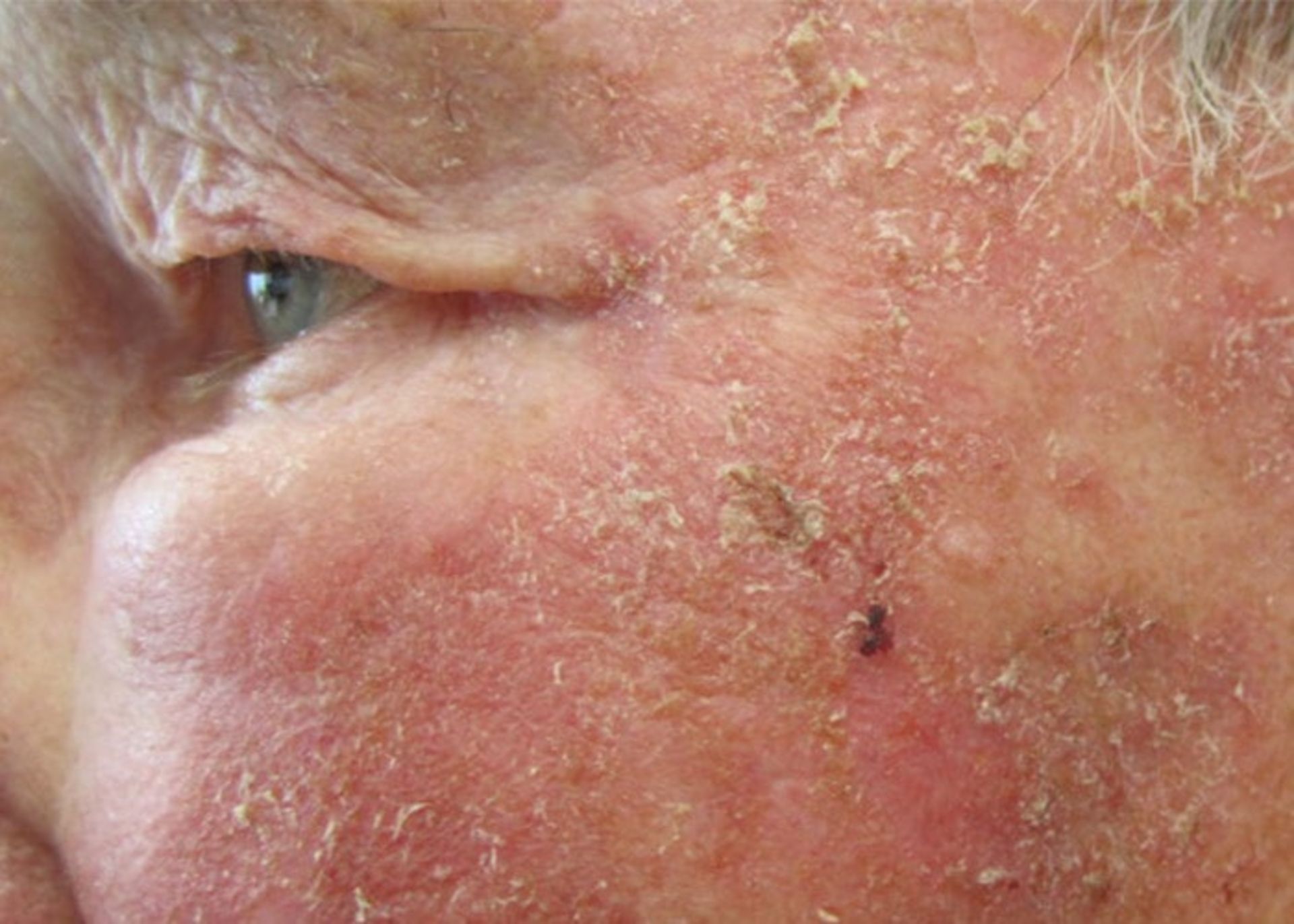
Actinic keratosis is a precancerous condition that can develop into squamous cell carcinoma if left untreated.
Symptoms of skin cancer
All skin cancers are not the same and may cause different symptoms. However, abnormal changes in the skin can be a warning sign for different types of skin cancer. Awareness of the condition of the skin can help diagnose the disease faster.
- Skin lesions: A new mole, abnormal growth, bump, sore, skin patch, or dark spot that appears and does not go away.
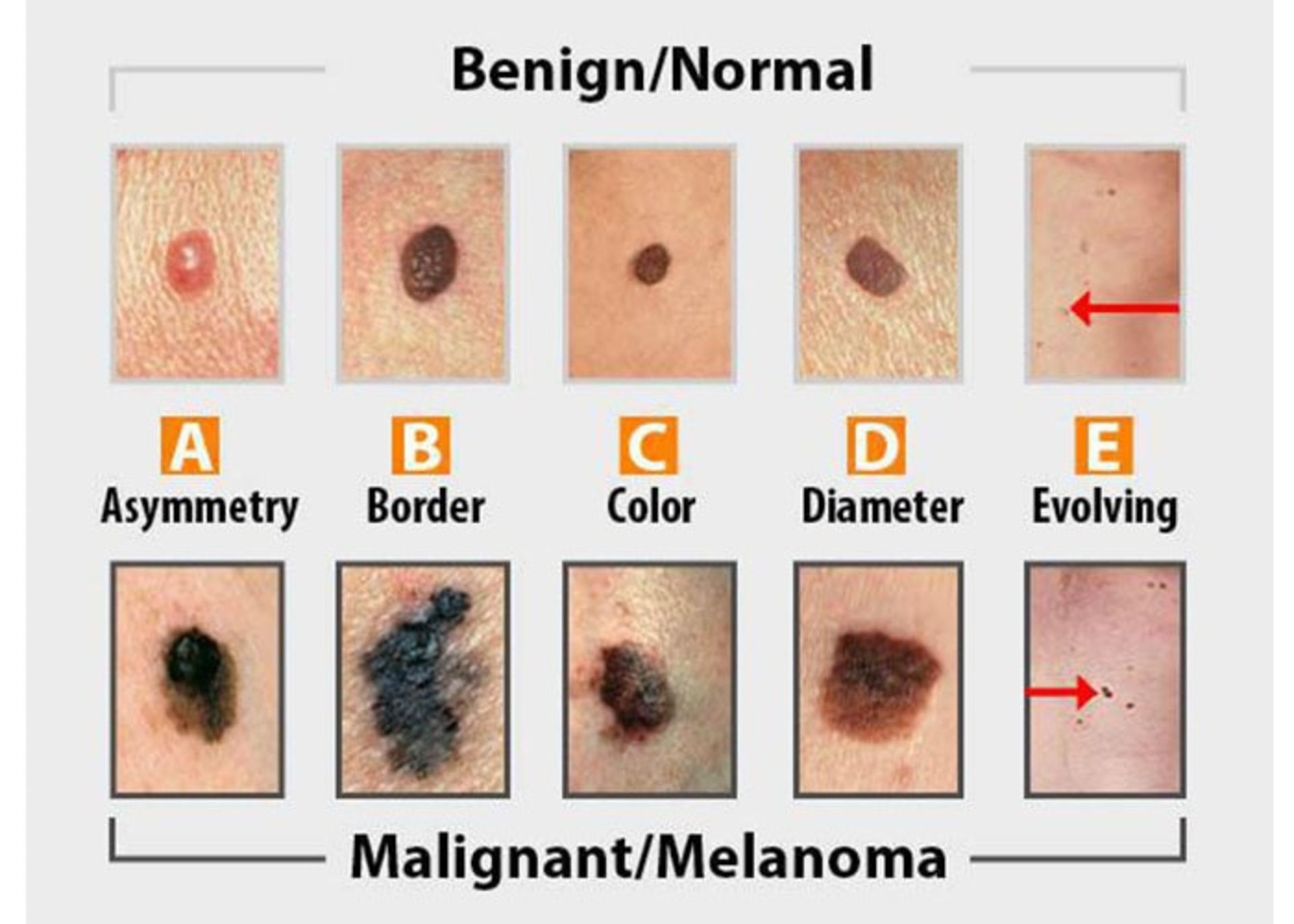
- Asymmetry: both sides of skin lesions or moles are not the same.
- Edges: The edges of the skin lesions are jagged and the edges are not equal.
- Color: The stain has an unusual color such as white, black, blue or red.
- Diameter: The thickness of the stain is more than half a centimeter.
- Change: A visible change in the size, color, or shape of a mole.
Moles and skin lesions that can be related to skin cancer often look like moles and skin spots that are not cancerous at all, so you must see a doctor for diagnosis.
Causes of skin cancer
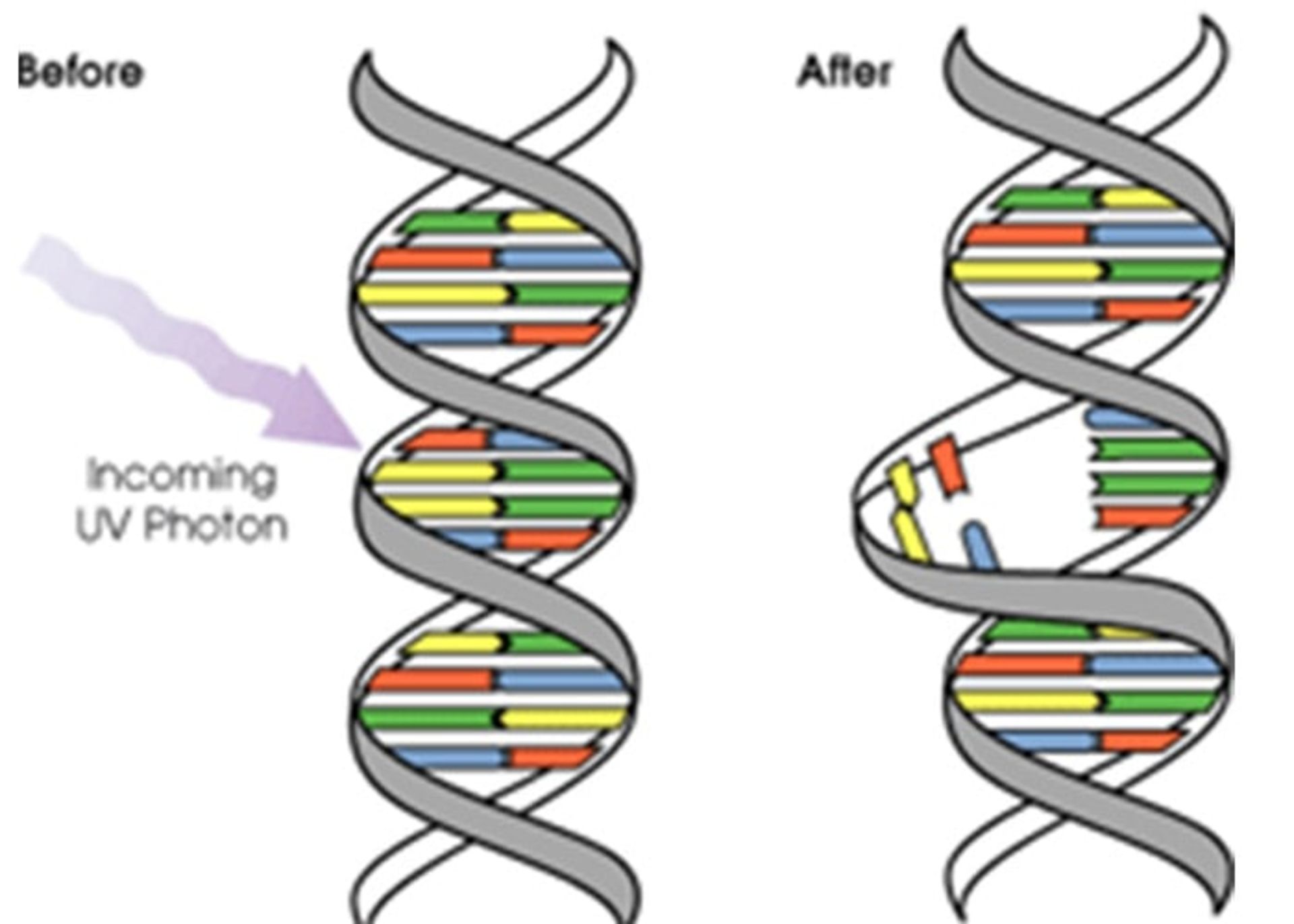
Both types of skin cancer occur when mutations occur in the DNA of skin cells. These mutations cause the skin cells to grow out of control and create a mass of cancer cells in the skin. Basal cell skin cancer is caused by ultraviolet (UV) rays from the sun or tanning devices. UV rays can damage the DNA in skin cells, which will result in abnormal cell growth. Squamous cell cancer is also caused by ultraviolet radiation. In addition, squamous cell cancer may also occur as a result of long-term exposure to cancer-causing chemical agents. This condition can manifest itself in wounds from burns or other injuries, and viruses such as the human papillomavirus (HPV) may cause it.
The cause of melanoma is not clear. Most moles don’t turn into melanoma, and researchers aren’t sure why in some cases they do become cancerous. Like basal cell cancer and squamous cell cancer, melanoma may be caused by UV radiation. However, melanoma can also develop in parts of the body that are not normally exposed to sunlight.
When is a mole dangerous or at high risk of becoming skin cancer?
Moles are usually harmless and rarely turn into skin cancer. If a mole becomes cancerous, it becomes melanoma. There is a precancerous stage called a dysplastic mole where the mole is no longer normal. An early sign of melanoma is a change in a mole: asymmetry, abnormal edges, changes in color, increased diameter, or other changes in condition can indicate that the mole has turned into melanoma. Moles never develop into basal cell carcinoma or squamous cell carcinoma. In the figure below, a normal mole (top row) is compared to a cancerous mole (bottom row).
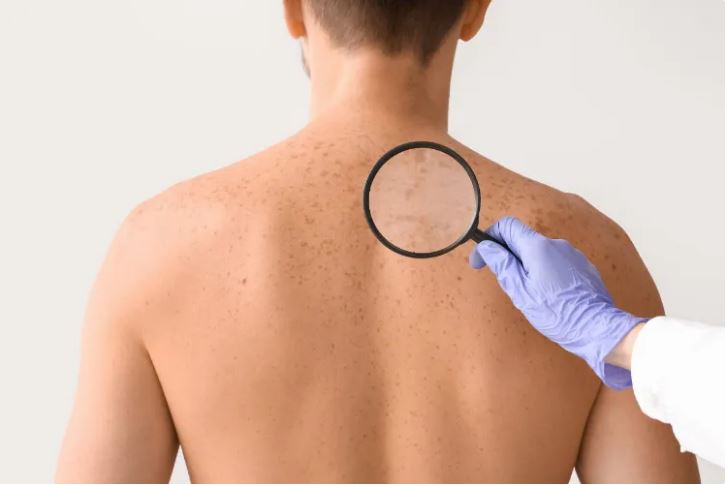
Skin cancer diagnosis
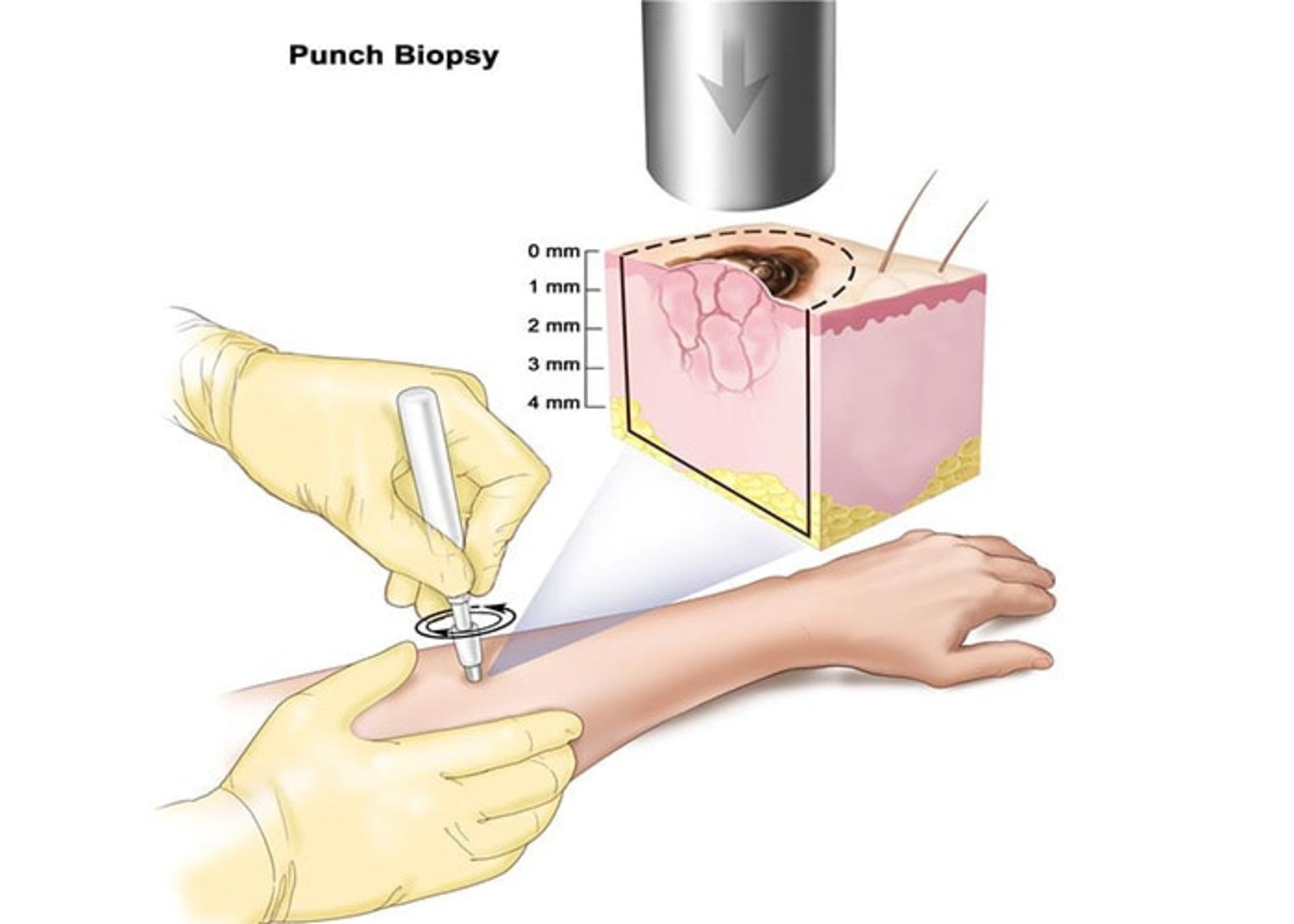
If a person notices suspicious spots and lumps on their skin or if they notice a change in spots that were already there, they should see a doctor. The specialist doctor examines the shape, size, color, and texture of the suspicious area on the skin, and if he suspects that the area is cancerous, he may perform a biopsy (tissue sampling). During this simple and safe procedure, a sample of suspicious tissue is isolated and examined. With this examination, it is determined whether a person has skin cancer or not. If a person is diagnosed with skin cancer, other tests may be needed to determine how far the disease has progressed. The patient’s treatment plan depends on the type, stage of the disease, and other factors.
Skin cancer screening
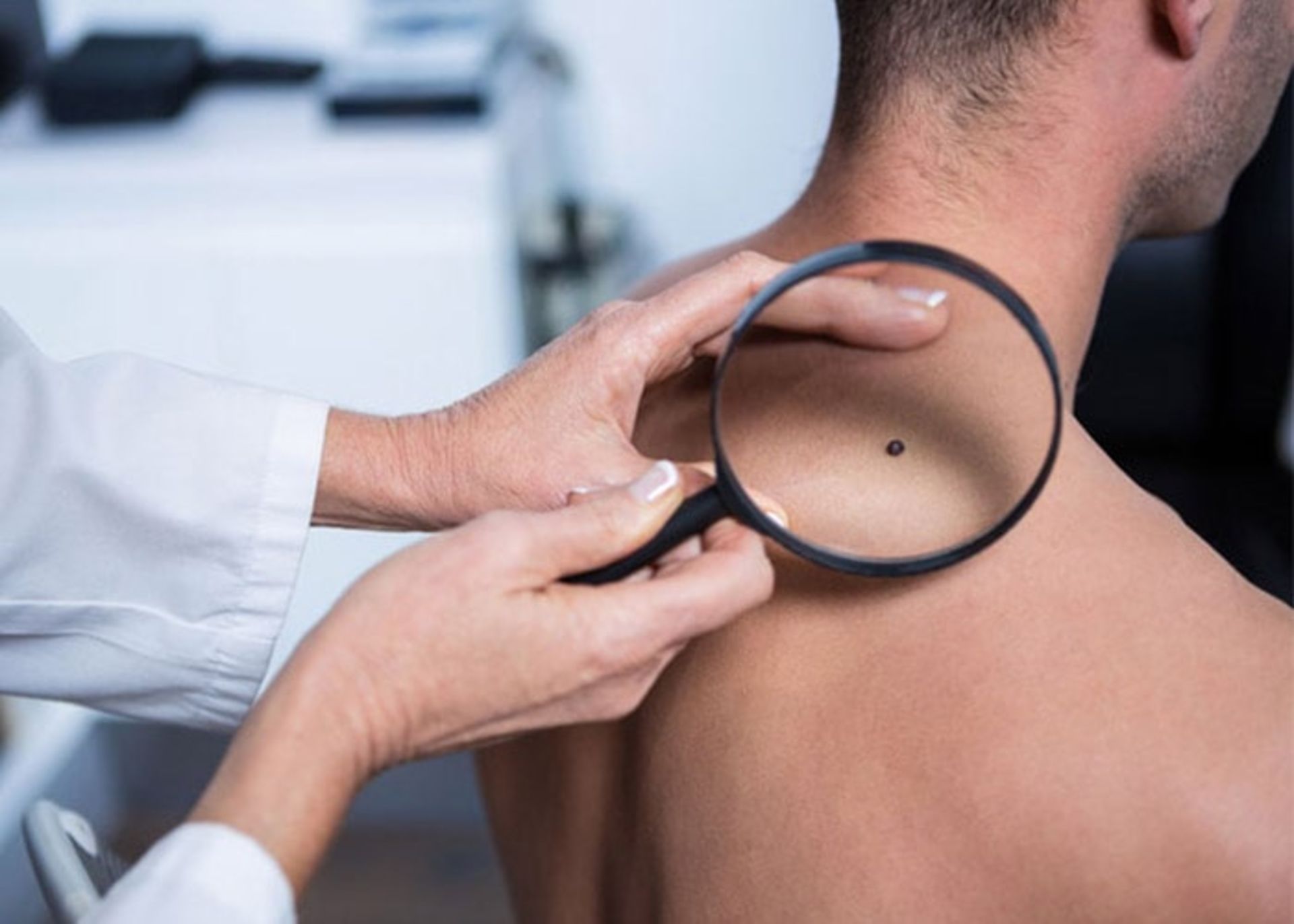
Screening for skin cancer by a dermatologist is quick and easy. The patient is asked to remove his clothes and the doctor looks all over his skin for unusual spots and moles. Early detection is the best way to ensure successful skin cancer treatment. Unlike other organs, the skin is always visible so that anyone can notice unusual changes or changes in the condition of their skin.
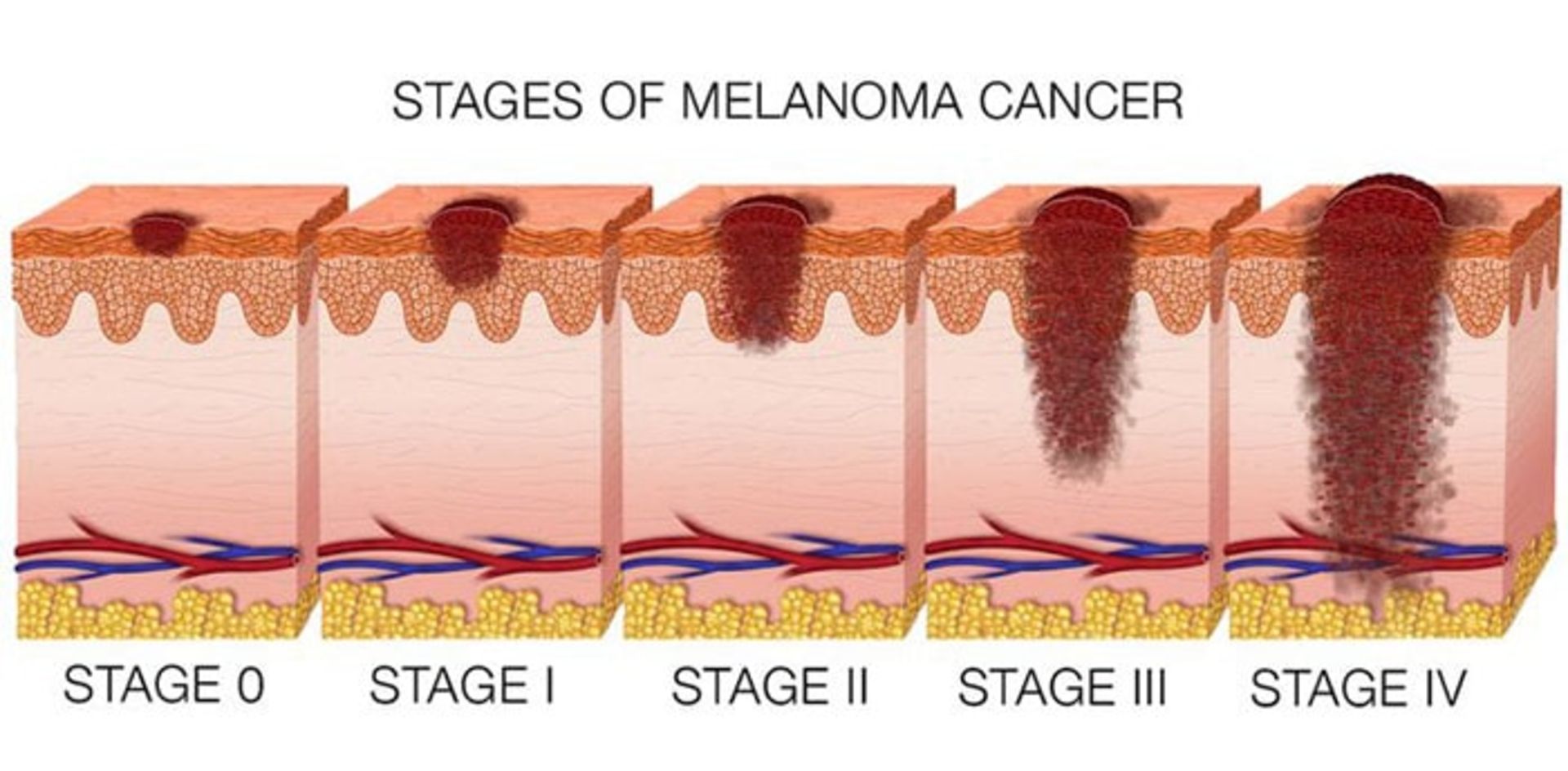
Stages of skin cancer
To determine the stage or severity of skin cancer, the doctor pays attention to the size of the tumor, and its status in terms of spreading to the lymph nodes and other parts of the body. Skin cancers are divided into two main groups in terms of stage of development: non-melanoma skin cancer and melanoma skin cancer.
Non-melanoma skin cancers include basal and squamous cell cancers, which have the following stages:
- Stage zero: Abnormal cells have not spread beyond the outer layer of the skin, the epidermis.
- Stage one: The cancer may have reached the next layer of the skin, the dermis, but its thickness is not more than two centimeters.
- Stage two: In this stage, the tumor has a size of more than two centimeters, but it has not yet reached the surrounding areas or lymph nodes.
- Stage three: The cancer has spread from the primary site to the adjacent tissue or bone and its size is more than three centimeters.
- Stage four: The cancer has spread to more distant areas than the primary tumor and has entered the lymph nodes, bone, and tissue. The size of the tumor is more than three centimeters.
Melanoma includes the following stages:
- Stage zero: This non-invasive type of skin cancer has not yet penetrated the lower part of the epidermis.
- Stage one: The cancer may have penetrated the second layer of the skin, the dermis, but its size is small.
- Stage two: The cancer has not spread beyond the primary tumor, but it is larger and thicker, and other signs and symptoms such as bleeding or scaling may also be seen.
- Stage three: The cancer has spread and entered the lymph nodes or adjacent tissue and skin.
- Stage four: It is the most advanced form of melanoma. In stage four, the cancer has spread beyond the primary tumor and is seen in lymph nodes, organs, or tissues far from the original cancer site.
If the cancer comes back after treatment, it is called recurrent skin cancer. There is a possibility of cancer returning in any person whose skin cancer has been diagnosed and treated, so it is necessary to conduct investigations in this field on these patients.
Skin cancer treatment methods
The treatment method for this disease depends on various factors such as the size, location, type, and stage of the disease. After considering these factors, the medical team may recommend one or more of the following treatments:
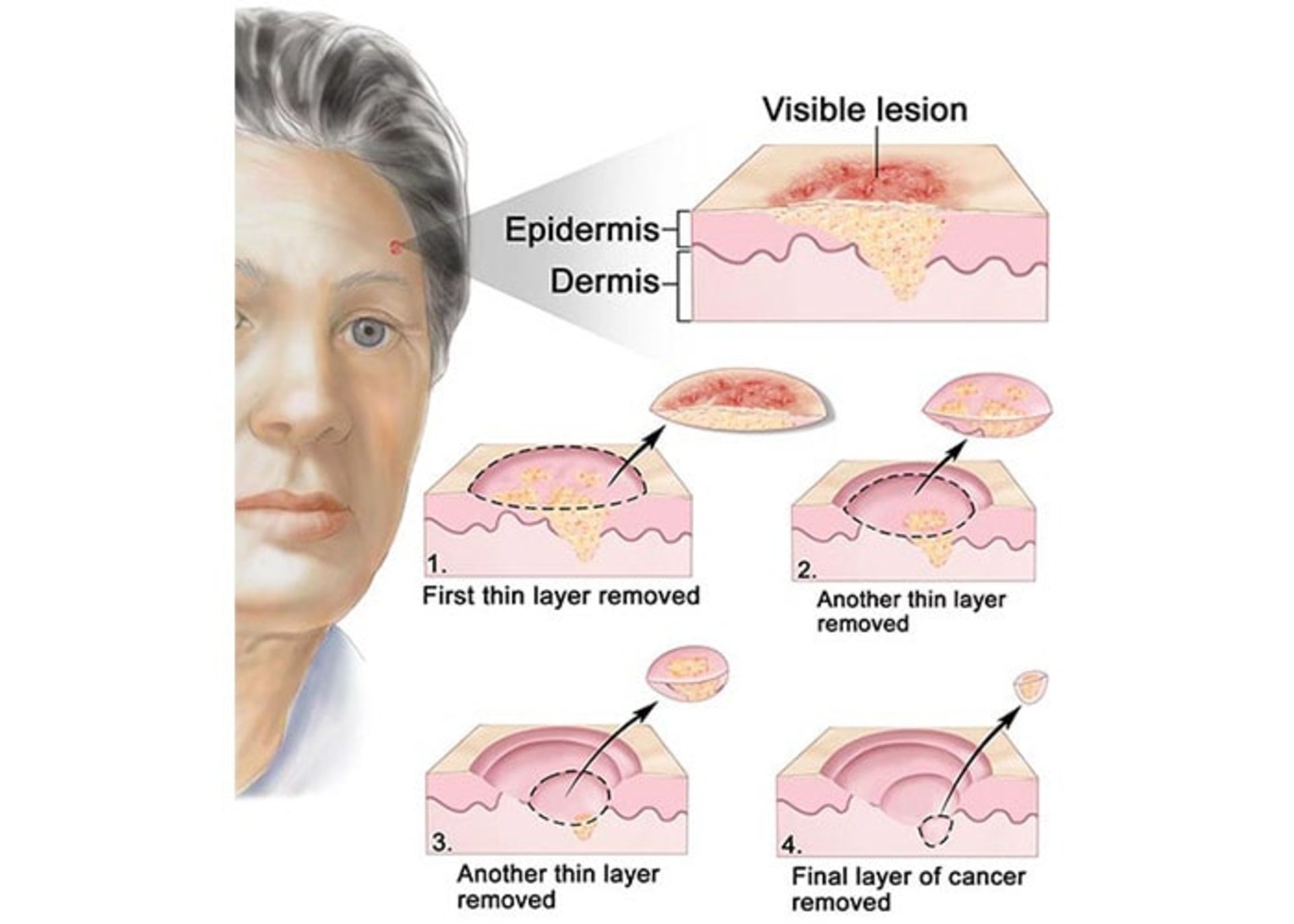
Micrographic Maze surgery: the desired area is locally numbed and the surgeon removes the tumor along with a small amount of surrounding healthy tissue. The tissue is quickly examined under a microscope and areas that still contain microscopic remnants of the tumor are removed and reexamined until no trace of tumor-related cells is seen (figure below). This complex and expensive treatment option is the treatment of choice in cases where it is critical to preserve the healthy tissues around the tumor, as well as in the cases where the borders of the tumor are not well defined, as well as in the cases of tumors that have been treated before but have recurred.
Tumor destruction by curettage and electrocautery (EDC): The tumor area is numbed using a local anesthetic and scraped repeatedly using a sharp blade, and then the edges of the wound are cauterized using an electric needle (electrode). The advantage of this method is that it is fast, easy, and relatively cheap. The problem with this method is that the scar is usually unpleasant and the probability of recurrence is 15%.
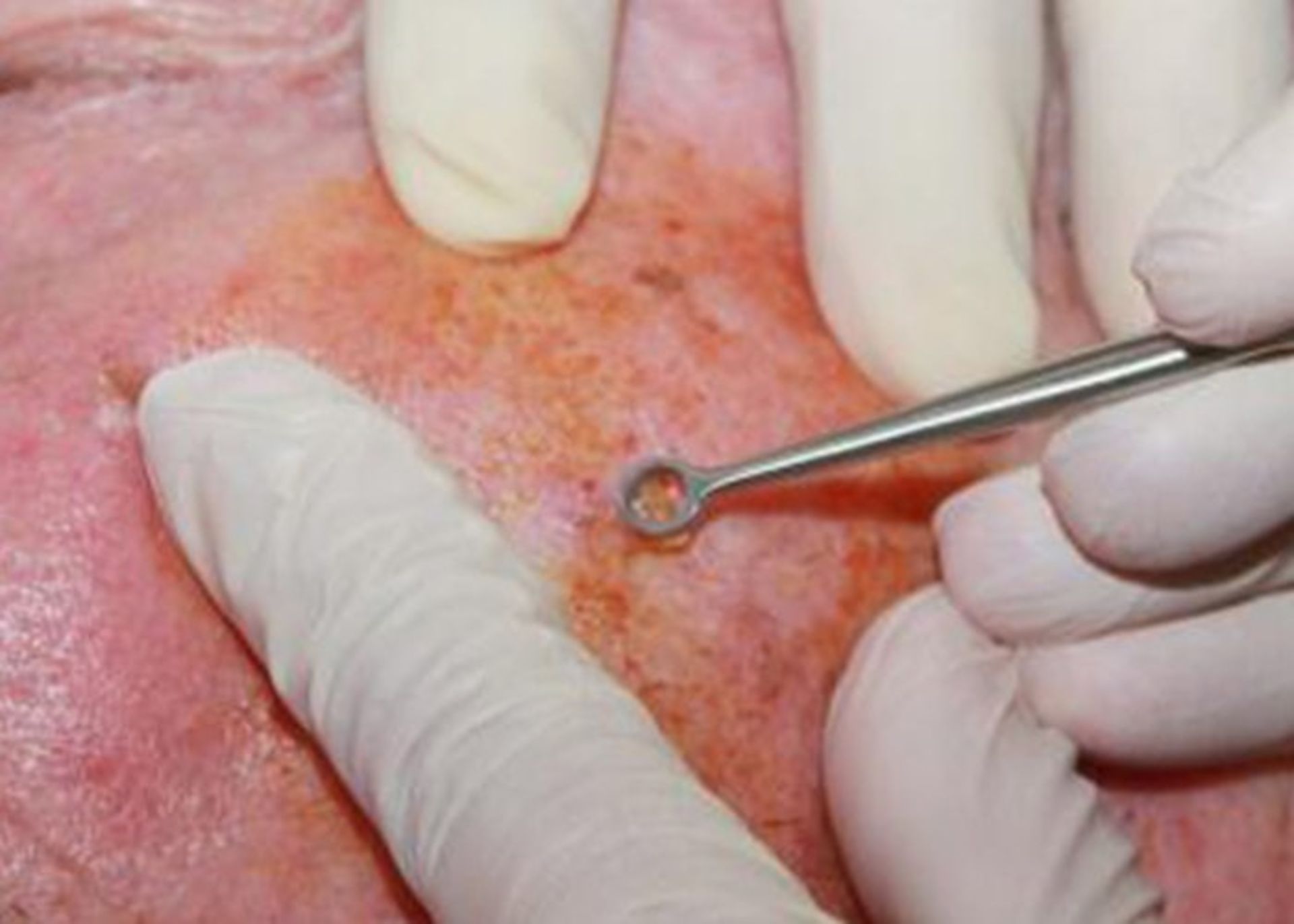
In the EDC method, first, the infected area is repeatedly scraped using a sharp blade to remove abnormal tissues.
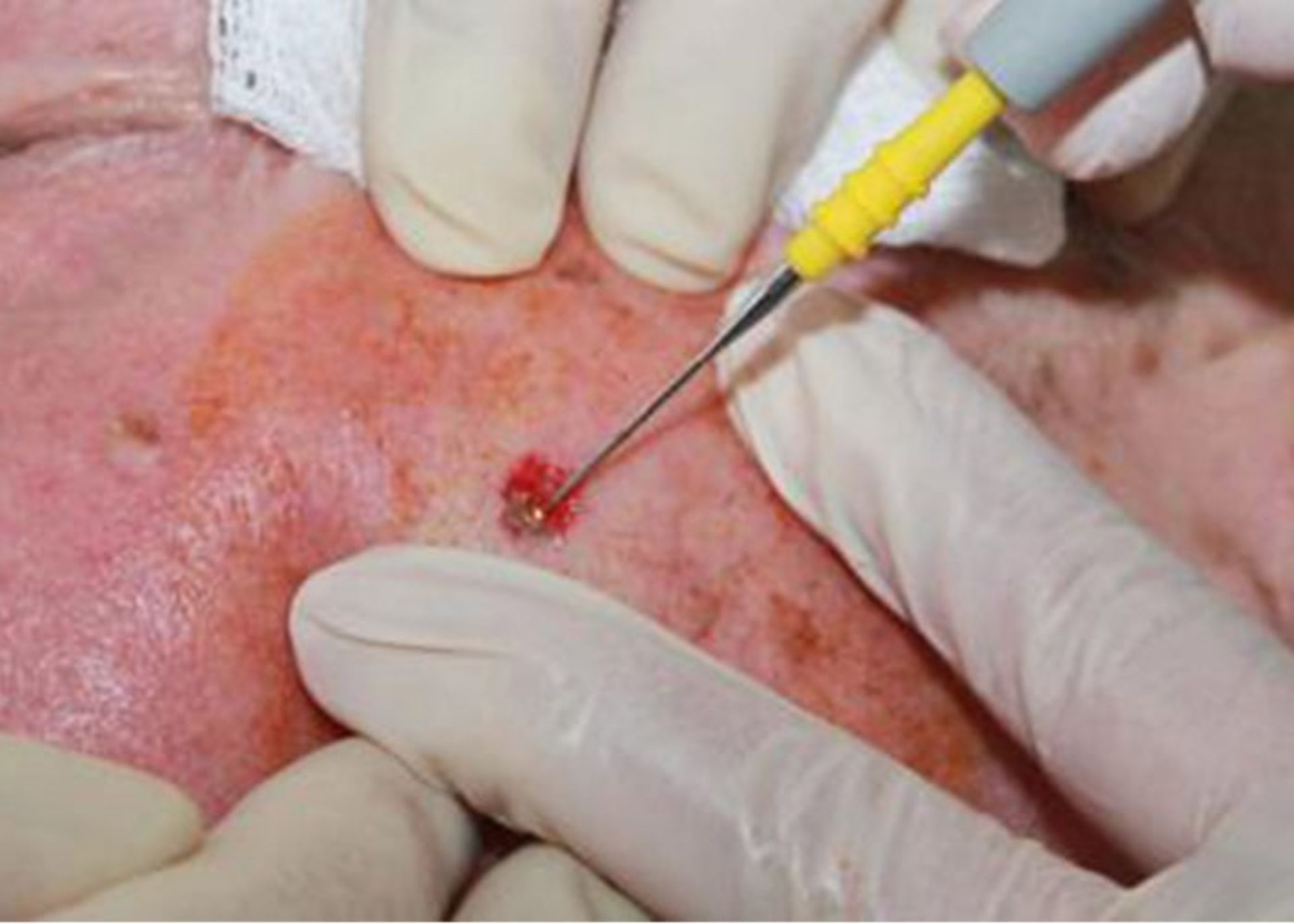
The edges of the wound are cauterized with an electric needle to remove the remnants of cancer cells.
Surgical tumor removal: First, the tumor area is numbed using a local anesthetic. A ball-like section of skin, which includes the tumor, is removed from the skin, and then the edges of the wound are sutured. In the case of large tumors, a skin graft may be needed to close the wound. The advantage of this method of treatment is that its success rate is more than 90% and the surgeon can simultaneously check whether the entire tumor has been removed or not. The wound created in this way is better than the wound produced by the EDC method, but it has more complexity and cost.
Radiation therapy: In this method, during 10 to 15 sessions, the tumor and a small amount of the surrounding skin area are exposed to high radiation. This form of treatment is useful in cases where patients cannot undergo surgery. The advantage of this method is that no cutting is done. One of the disadvantages of this expensive method is that the treated area cannot be examined at the same time to determine whether the entire tumor is gone or not, and the wounds caused by this method may worsen over time. This method is usually used for elderly people.
Drug therapy: In the case of superficial basal cell cancer, some creams, gels, and solutions such as Imiquimod (Aldara) can be used. The way these drugs work is to stimulate the patient’s immune system to produce interferon. Interferons are proteins that attack cancer cells. Another effective drug in this field is fluorouracil (5-FU), which is a chemotherapy drug. Some patients do not experience any side effects from these topical treatments, but some may experience redness, inflammation, and skin irritation. One of the disadvantages of topical drugs is that because no tissue is available for examination, it is not possible to determine whether the tumor has been completely removed or not.
Cryotherapy: In this method, the tissue is frozen and destroyed using liquid nitrogen. The doctor sprays liquid nitrogen on the infected area and some of the surrounding areas. This causes a burning or stinging sensation in the person, which disappears within a few minutes. Liquid nitrogen freezes and kills the abnormal cells, creating a sore that will be red for several days and may blister and ooze. The area may be swollen for a few days, and in some cases this may need to be repeated. A crust will form over the wound and the dead tissue will fall off within one to four weeks, depending on the size of the treated area. After that, healthy skin cells grow and a scar may remain. Healing may take several weeks, and the healed skin will likely be paler than the surrounding area.
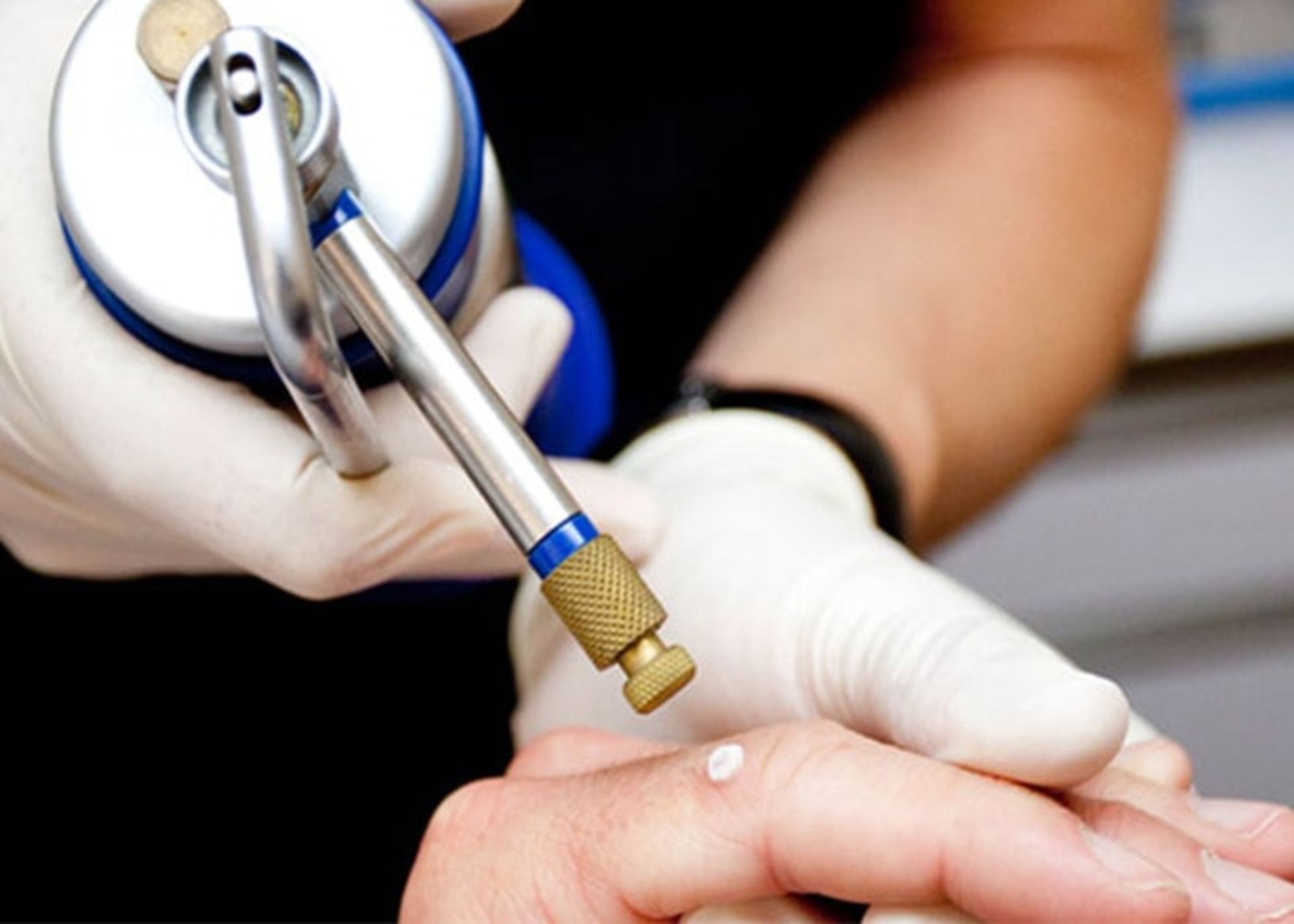
In the cryotherapy method, liquid nitrogen is used to freeze and destroy tissue
Photodynamic therapy (PDT): In this method of treatment, a drug and a special type of light are used. The drug is a chemical that sensitizes the skin to light. This drug is called a photosensitizing agent. The light can be a laser or another type of light. The drug can be used as a cream on the skin or injected into the bloodstream. By absorbing the drug, the cells become sensitive to light. Then the doctor shines light on the desired area and destroys the cancer cells.
Prevention of skin cancer
To reduce the risk of skin cancer, long-term exposure to sunlight and other sources of ultraviolet radiation should be avoided. for example:
- Avoiding tanning devices and solar lamps
- Avoid exposure to sunlight when the intensity of the radiation is high, otherwise you should use an umbrella.
- Using face and lip sunscreens with SPF of 30 or more
- Using a brimmed hat and protective clothing in the sun
- Use sunglasses that have 100% protection against UVB and UVA.
It is also important to check your skin regularly to see if there are any lesions on the skin or if the previous spots and moles have changed.

Skin cancer risk factors
Certain factors increase the risk of skin cancer. For example, a person is more likely to develop skin cancer if:
- Have a family history of skin cancer.
- exposed to certain substances such as arsenic compounds, radium, bitumen, and creosote.
- exposed to radiation, for example, during special treatments for acne and eczema.
- exposed to excessive sunlight, tanning lamps, and other similar factors.
- Live in sunny, hot climates or high altitudes.
- Be constantly engaged in outdoor work.
- Have a history of severe burns.
- They have many large and abnormal moles.
- The skin is pale and has freckles.
- Have skin that gets sunburned easily.
- Have natural blonde or red hair.
- Have blue or green eyes.
- Have precancerous skin lesions.
- Have a weak immune system, for example, have HIV infection.
- Have a history of organ transplantation and taking immunosuppressive drugs.
The use of sunglasses with 100% UVB and UVA protection is recommended to prevent skin cancer.
Doctors who treat skin cancer
If a person is diagnosed with skin cancer, a group of doctors may help the patient with different aspects of the condition. for example:
- A dermatologist who treats skin diseases.
- A cancer surgeon who treats cancer through surgery.
- A radiation therapy specialist who treats cancer using radiation therapy.
- A cancer drug treatment specialist who treats cancer using targeted drug therapies, immunotherapy, chemotherapy, or other drugs.
Complications and complications related to skin cancer
Side effects of skin cancer include the following:
- Disease recurrence means the return of cancer after treatment
- Local recurrence means the spread of cancer to nearby tissues
- Cancer progression in which cancer cells spread to the muscles, nerves, or other organs of the body.
If a person has skin cancer, there is a high risk that the disease will reappear in another part of his body.
Is skin cancer hereditary?
Since most types of skin cancer are caused by exposure to ultraviolet light, they are generally not considered hereditary skin cancers. But the fact that skin cancer is more common among people with less skin pigmentation, and skin color trait is an inherited trait, so it can be said that the role of genetics is important. There are several rare genetic syndromes that lead to an increased frequency of skin cancer cases among those with these syndromes. There is a type of hereditary melanoma called FAM-M syndrome, which is caused by a mutation in the CDKN2A gene located on chromosome 9. This mutation is also called the p16 mutation.
What is the prognosis and longevity of skin cancer?
In general, the prognosis for non-melanoma skin cancer is excellent. Both basal cell carcinoma and squamous cell carcinoma are highly treatable. Almost no death occurs from basal cell cancer, and rarely, death can occur from squamous cell cancer, which is mainly in people who are immunosuppressed. Depending on the treatment method and the location and type of skin cancer, the probability of recurrence of a skin cancer treated in the maze surgery method is 1-2% and in the electric burning and curettage method it is about 10-15%. Early detection of skin cancers can lead to better treatment outcomes.
The results of new research related to effective factors on skin cancer
Statistics show strong evidence that drinking water contaminated with arsenic compounds increases the risk of skin cancer. Arsenic contamination is common in underground water and arsenic contamination of drinking water is significant in some areas. At least 140 million people in 50 countries of the world are consuming drinking water whose concentration of arsenic compounds exceeds the set limit (10 micrograms per liter). Arsenic is one of the natural compounds of the earth’s crust and is widely present in the air, water, and soil. This substance is very toxic in its inorganic form. People are exposed to high levels of inorganic arsenic through drinking contaminated water, using contaminated water to prepare food and irrigate agricultural crops, industrial activities, eating contaminated food, and using tobacco. Long-term exposure to inorganic arsenic, mainly through consumption of contaminated food and water, can lead to chronic arsenic poisoning. Skin lesions and skin cancer are some of the undesirable effects of this substance. In Iran, few researches have been conducted in the field of arsenic contamination of drinking water in different regions. In a study conducted in Ardabil City in 2017, it was found that the concentration of arsenic in the drinking water of this region is higher than the standard (with an average of 15 micrograms per liter). In a similar study in Kashmir city, the presence of high concentrations of arsenic compounds in drinking water was confirmed.
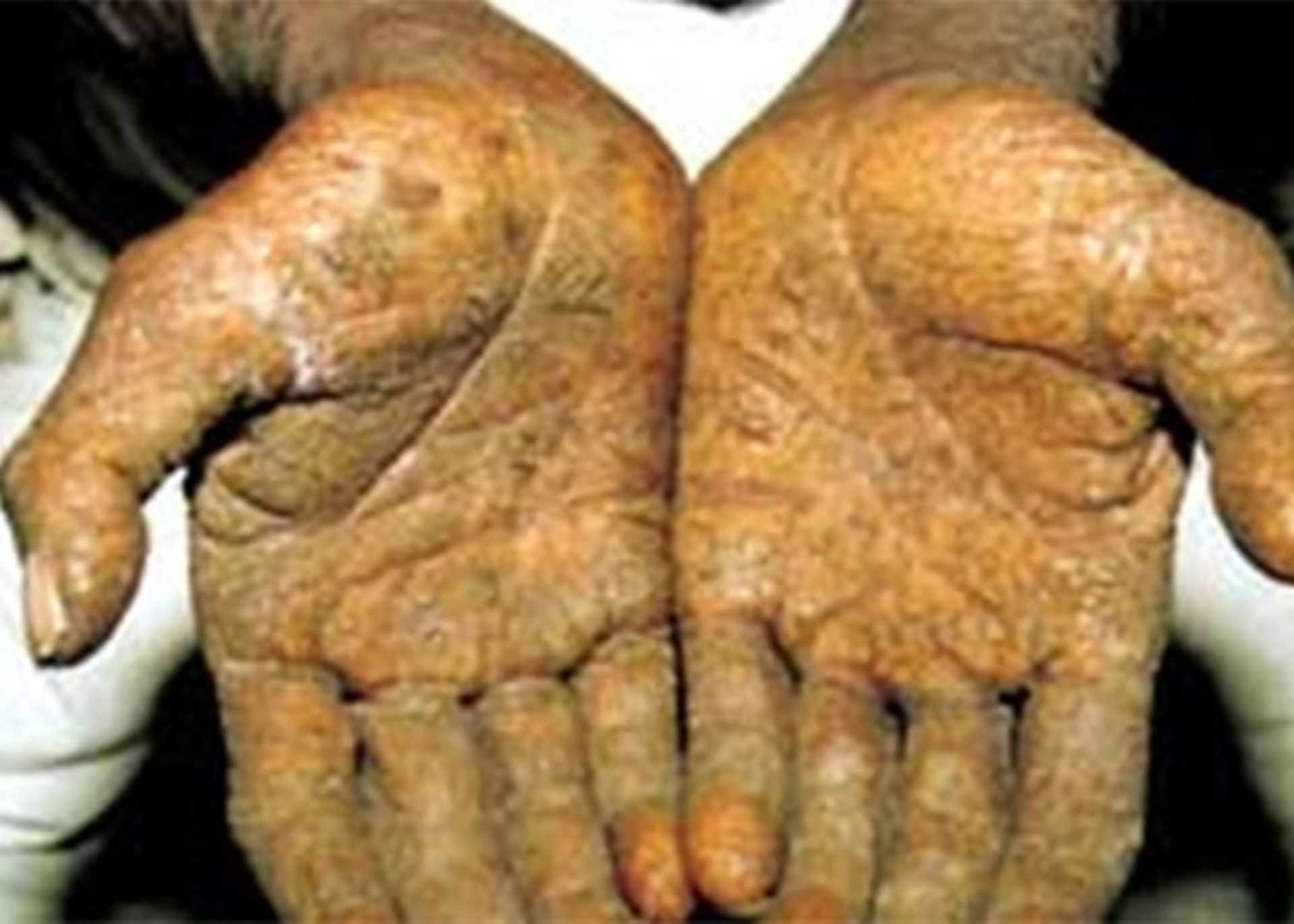
Skin lesions and skin cancer are the main characteristics of arsenic pollution
Being taller at puberty also increases the risk of developing malignant melanoma. Beta-carotene supplementation has no significant effect on increasing the risk of non-melanoma skin cancer. Drinking coffee may reduce the risk of malignant melanoma in women and may reduce the risk of basal cell carcinoma in men and women. There is also evidence that drinking alcoholic beverages may increase the risk of malignant melanoma and basal cell carcinoma.
How common is skin cancer?
In general, melanoma skin cancer is the 15th most common cancer among men and women. The prevalence of both melanoma and non-melanoma skin cancer has increased in recent decades. Currently, about 2 to 3 million cases of non-melanoma skin cancer and 132,000 cases of melanoma skin cancer occur worldwide every year. One out of every three cancers diagnosed is skin cancer, and one in five Americans will develop skin cancer in their lifetime. In 2018, 300,000 new cases of this cancer have been diagnosed. Australia, New Zealand, Norway, Denmark, and the Netherlands are among the countries in the world with the highest prevalence of this disease.
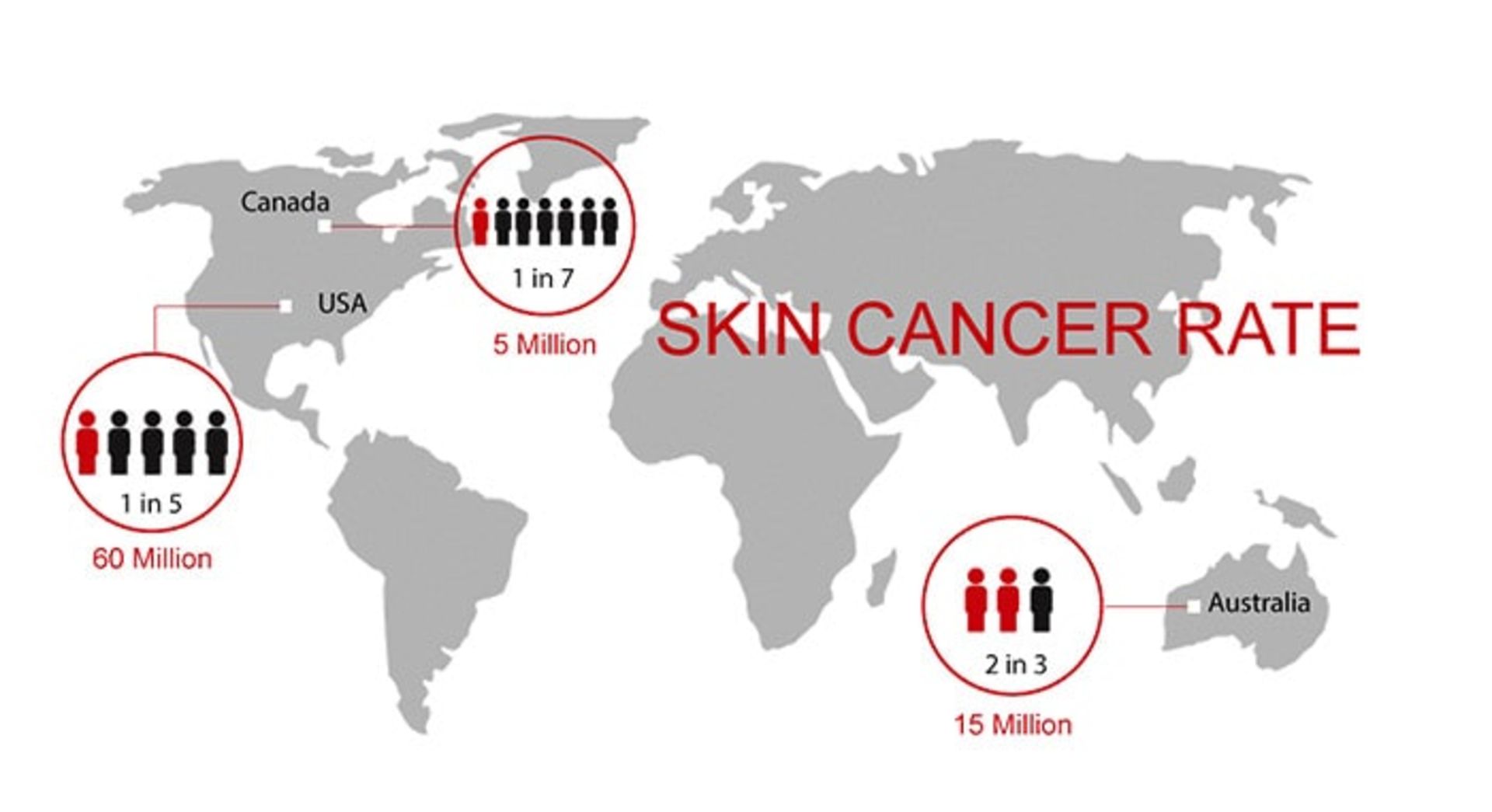
With the process of destroying the ozone layer, the atmosphere loses its protective layer and more ultraviolet rays reach the earth’s surface. It is estimated that a ten percent decrease in the ozone layer will lead to the occurrence of 300,000 cases of non-melanoma skin cancer and 4,500 cases of melanoma skin cancer. The global prevalence of melanoma is increasing, although the main predisposing factors for the development of melanoma appear to be related to recreational exposure to sunlight and having a history of sunburn. The responsibility for these factors lies with the people themselves.

The strange ways skin affects our health
Worn-out or unhealthy skin is a major contributor to every age-related disease, from Parkinson’s to type 2 diabetes. BBC journalist Zarya Gorot explains how skin affects health and how to protect it.
I am boating in the Ardèche Strait in the south of France when I notice people’s strange looks. It is early afternoon on a scorching July day and the sky is blue and clear. Although there are high cliffs on both sides of the river, I have never felt the sun’s rays so strongly.
The sun’s rays have turned the surface of the water into a squiggly path of brilliant light that is impossible to look at. I have chosen my outfit with the seriousness of an explorer who is going to walk in the African desert. My clothes cover my whole body and protect me from the sun. I used a wide-brimmed fishing hat as well as plenty of high SPF sunscreen and I didn’t forget my sunglasses. I am determined to prevent further aging from the sun. But are there other hidden benefits to these extreme measures of mine?
The latest research shows that our skin is not just a mirror of our lifestyle that reflects the effects of years of smoking, drinking alcohol, and stress. According to the new view, the skin as the largest organ of the body is an active participant in our physical health, and wrinkled and dry skin itself causes aging.
Weird theory
In 1958, the Baltimore Longitudinal Study began, which was supposed to be a scientific study of aging with a bold and rather unorthodox hypothesis. Before that, scientists used to study donated cadavers to understand the physiology of living people. But this time the subjects were examined while their hearts were still beating and their bodies were fully alive. Researchers have followed thousands of men and women for decades to study how genes and environment affect their health.
Wrinkled and dry skin causes aging
In the two decades since the Baltimore study began, scientists have made interesting advances: from the discovery that men who were emotionally unstable were more likely to develop heart disease to the discovery that our problem-solving abilities decline slightly as we age. .
One of the most striking findings of the Baltimore study confirms what researchers have long suspected: how young you look is an accurate measure of how healthy you are inside. In 1982, researchers found that men who looked much older than their age at the start of the study were more likely to die.
In more recent studies, 99% of patients who looked at least 10 years older than their actual age had health problems. It appears that skin health can be used to predict a number of seemingly unrelated factors, from bone density to the risk of developing neurodegenerative diseases or death from cardiovascular disease. But is the skin merely a sign of damage that has accumulated in us, or is it something more complex: can it preserve the health of the healthy and worsen the condition of the unhealthy?
Chronological age and biological age
There are two main ways to measure people’s age. The first method is the standard method known as chronological age. But there is also biological age, which shows the speed of aging of the body. Biological age may vary between different people and even within the same body.
As we age, our chronological age eventually affects our appearance: skin becomes thinner and more uneven, and its elasticity decreases; Because the cells responsible for the production of pigment and collagen die or get old. But usually, the environment causes real damage to the skin.
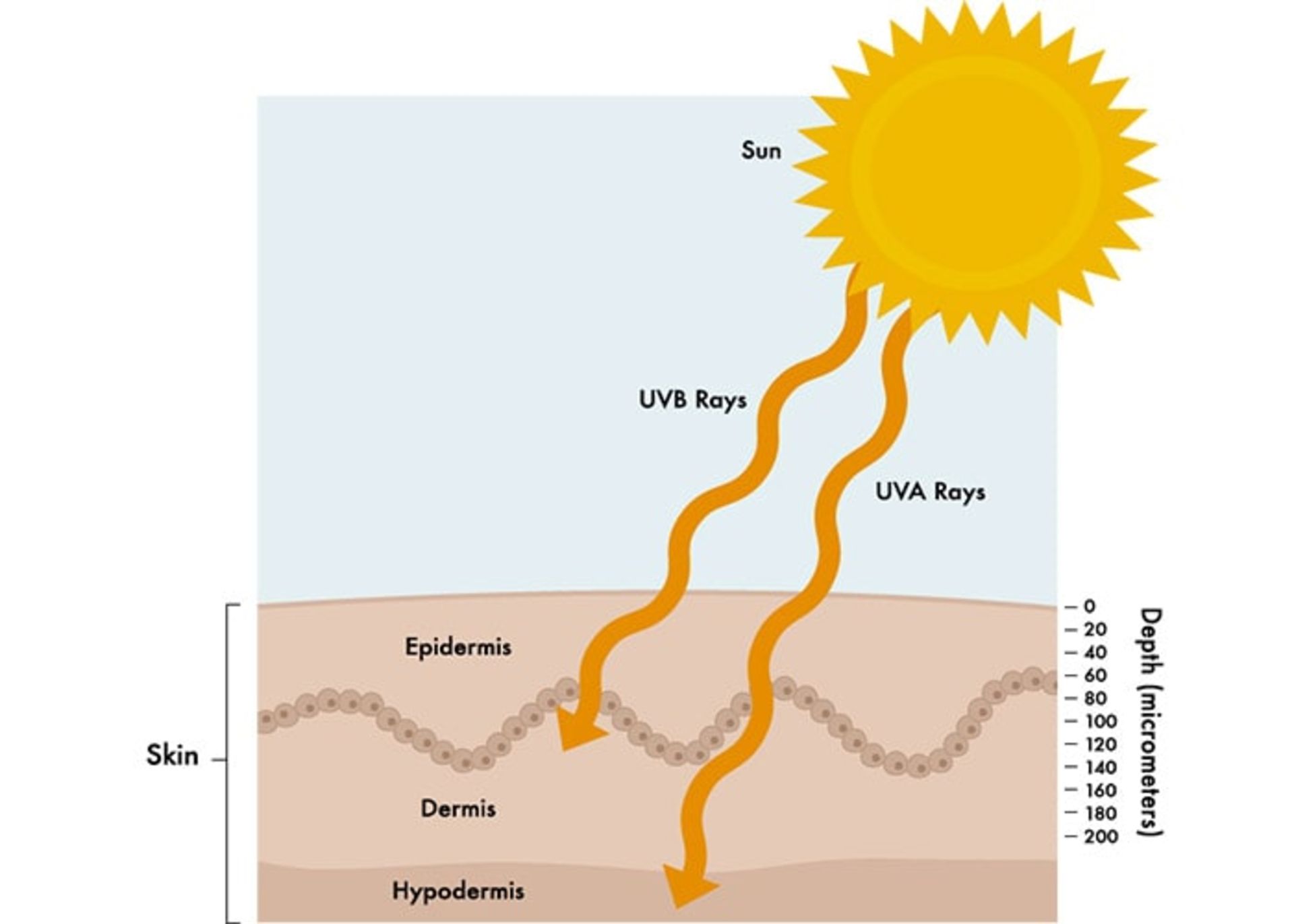
Although UVB rays can damage our DNA and cause sunburns, mutations, and skin cancer, 95% of all UV radiation reaching the Earth’s surface is UVA. This part of the sun’s rays has a longer wavelength and can penetrate deep into the skin, break down collagen, and stimulate cells to produce melanin.
At the microscopic level, skin that has aged due to exposure to sunlight is thicker and has malformed collagen and elastin fibers. On the visible surface, such skin has an uneven color and is significantly more wrinkled.
Even the darkest skin can burn and is susceptible to photoaging, although it takes longer for wrinkles to appear.
 While UVB rays usually damage the surface of the skin, UVA penetrates deep into the skin and both can cause systemic inflammation.
While UVB rays usually damage the surface of the skin, UVA penetrates deep into the skin and both can cause systemic inflammation.
Internal factors are thought to be responsible for a small part of skin aging, while UV light is responsible for more than 80% of visible skin changes.
Along with the physical effects described, the skin also undergoes a chemical transformation, and this is something that may have a profound effect on our general health.
Inflammatory aging
In 2000, a group of scientists from the University of Bologna in Italy proposed a new way of thinking about aging by observing how organisms react to stress.
In a healthy young person, the immune system normally functions to maintain order, that is, to repair damage and fight off infections. But when we age or when our health is not good, these inflammatory responses can cross a certain threshold and trigger the release of a cascade of powerful chemicals that travel throughout the body, destroying healthy cells and breaking DNA.
Even the darkest skin is susceptible to sun aging and can burn
The term inflammatory aging is used to describe the global inflammation that accompanies the aging process. Research shows that wrinkled, diseased, or damaged skin becomes part of the inflammatory system and releases chemicals that cause further damage and inflammation.
Higher levels of inflammatory cytokines and chemokines are observed in aged skin. These chemicals destroy collagen and elastin and cause thinning, wrinkling, and loss of skin elasticity. They also disrupt the skin barrier, increasing water loss and susceptibility to stressors. This feedback loop combines with aging cells in the skin, which in turn release their own inflammatory chemicals. Chemicals released by unhealthy skin enter the bloodstream and from there reach different tissues and damage them. The result of this is accelerated aging and a higher risk of various diseases.
So far, old or diseased skin has been associated with the onset of cardiovascular disease, type 2 diabetes and cognitive disorders, as well as Alzheimer’s and Parkinson’s disease.
The importance of skin protection
The first step to protecting your skin is to avoid the sun. In order to protect the skin, observe the following:
- Wear protective clothing against sunlight.
- Use sunscreen with a high protection factor.
- Wear a brimmed hat.
- Use sunglasses.
- Do not stay in the sun as much as possible.
Protecting the skin from the sun is very effective in preventing the visible signs of aging. In a preliminary study, those who used a broad-spectrum sunscreen with an SPF of 15 every day for four and a half years showed no signs of further skin aging.
 Moisturizing the skin reduces inflammation.
Moisturizing the skin reduces inflammation.
The important thing in choosing a sunscreen is to choose a product that is broad-spectrum. Broad-spectrum sunscreen not only absorbs or reflects UVB (indicated by SPF) but also protects against UVA. Dermatologists recommend that you always check the product label for UVA protection. Protection against UVA is usually indicated by UV-PF or PPD.
Sunscreen can prevent inflammation that occurs when the skin is exposed to the sun, and as previously mentioned, inflammation is the first step toward aging-related diseases. But using sunscreen is not the only way to maintain skin health.
The easiest way to improve skin health is to moisturize it. Moisturizing the skin reduces inflammation and may even help prevent dementia.
In addition to uneven color and wrinkling, skin that has aged due to exposure to sunlight and age is drier. The moisture level of human skin reaches its peak at the age of 40, and after that, it decreases drastically and produces less amounts of natural moisturizers, namely lipids, filaggrin, sebum, and glycerol.
Dry skin is problematic because when the skin is dry, its function as a barrier between the inside and the outside of the body is weakened. When our skin is dry and scaly, its natural functions (keeping out infectious agents, environmental toxins, and allergens while maintaining moisture) become more difficult.
 Sun-damaged skin releases chemicals that contribute to systemic inflammation and increase the risk of age-related diseases.
Sun-damaged skin releases chemicals that contribute to systemic inflammation and increase the risk of age-related diseases.
Adding moisture to the skin is not a complicated task, and this simple intervention produces significant results.
A group of researchers asked elderly volunteers to use a topical moisturizer twice a day for a month. Compared to older participants who did not use moisturizer, their skin was significantly repaired and their skin levels of inflammatory chemicals were lower.
Even the simplest moisturizers can help prevent inflammatory aging
The promising results of the above study were followed by another study in which people over the age of 65 used a moisturizing cream twice a day for three years. The cognitive performance of the participants was measured at the beginning and at the end of the study. After three years, the cognitive performance of the participants in the control group had declined significantly, but the cognitive performance of the group that hydrated their skin had not.
Read more: Inventing a new drug to treat influenza
Dry skin usually has a higher level of inflammation and is often itchy. A decrease in the level of hydration of the stratum corneum (the outer layer of the epidermis) probably plays a major role in inflammatory aging. On the other hand, scratching the skin intensifies the inflammation.
Natural ingredients include glycerol, petroleum, hyaluronic acid, and lipids that are normally found in the outer layer of the skin and are also the natural components of the most basic moisturizers. Drinking more water may also help hydrate the skin, although the evidence is unclear.
To visualize how much skin can affect the rest of your body, think about how much skin you have. There is as much skin on the inside of your body as there is on the outside of your body. When skin is damaged, every inch of it can release toxic chemicals. Therefore, protecting the skin from the sun is a very effective solution, but don’t forget to use moisturizer as well.
Conclusion
The skin not only indicates the internal state of our body and our lifestyle but also plays a role in age-related diseases. When the skin is exposed to environmental factors, especially sunlight, in addition to changes in appearance, it undergoes chemical changes and contributes to various diseases by participating in global inflammation.
Use a broad-spectrum sunscreen to protect your skin from the sun. Using a moisturizer also helps prevent and reduce inflammation and prevent skin damage.


What are the obstacles on the way for humans to reach Mars?


Unveiling of OpenAI new artificial intelligence capabilities


Samsung S95B OLED TV review


Discovery of the brain circuit that manages inflammation


History of the world; From the Big Bang to the creation of the planet Earth


Xiaomi Pad 6S Pro review


The biography of Pavel Durov


Is Telegram really safe?


AI PC; revolutionary technology of the future?


Black holes may be the source of mysterious dark energy
Popular
-



 Technology10 months ago
Technology10 months agoWho has checked our Whatsapp profile viewed my Whatsapp August 2023
-



 Technology11 months ago
Technology11 months agoHow to use ChatGPT on Android and iOS
-



 Technology10 months ago
Technology10 months agoSecond WhatsApp , how to install and download dual WhatsApp August 2023
-



 Technology11 months ago
Technology11 months agoThe best Android tablets 2023, buying guide
-



 AI1 year ago
AI1 year agoUber replaces human drivers with robots
-


 Humans1 year ago
Humans1 year agoCell Rover analyzes the inside of cells without destroying them
-



 Technology11 months ago
Technology11 months agoThe best photography cameras 2023, buying guide and price
-


 Technology11 months ago
Technology11 months agoHow to prevent automatic download of applications on Samsung phones
